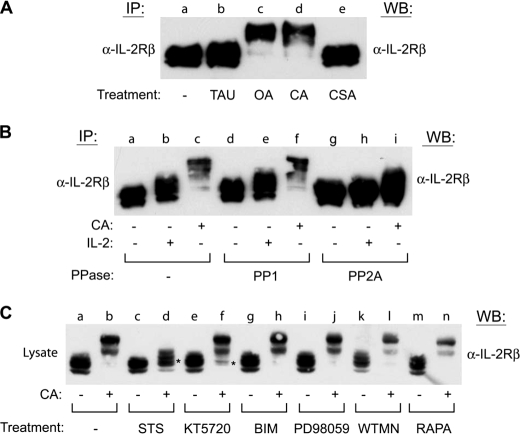
FIGURE 6.
PP2A directly regulates IL-2Rβ serine phosphorylation by a staurosporine-sensitive kinase. A, YT cells were incubated in the absence (lane a) or presence of TAU (1 μm) (lane b), OA (250 nm) (lane c), CA (100 nm) (lane d), or CSA (250 nm) (lane e) for 60 min. IL-2Rβ was immunoprecipitated (IP), separated by SDS-PAGE, and analyzed by Western blot (WB) as indicated. B, YT cells were incubated in the absence (lanes a, b, d, e, g, and h) or presence (lanes c, f, and i) of CA (100 nm) for 60 min prior to stimulation with IL-2 (lanes b, e, and h) for 10 min. IL-2Rβ was immunoprecipitated and left untreated (lanes a–c) or subjected to dephosphorylation using purified PP1 (lanes d–f) or PP2A (lanes g–i) for 60 min at 37 °C before separation by SDS-PAGE and Western blot analysis as indicated. C, YT cells were left untreated (lanes a and b) or treated with staurosporine (500 nm) (lanes c and d), KT5720 (5 μm) (lanes e and f), bisindoylymaleimide II (BIM) (250 nm) (lanes g and h), PD98059 (250 μm) (lanes i and j), wortmannin (50 μm) (WTMN) (lanes k and l), or rapamycin (100 nm) (RAPA) (lanes m and n) for 60 min at 37 °C prior to treatment with or without CA (100 nm) for an additional 60 min. Cells were lysed and analyzed by Western blot with α-IL-2Rβ. * indicates inhibition of CA altered electrophoretic mobility shift. Representative data from three independent experiments are shown.