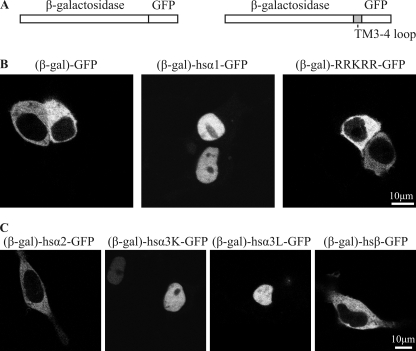
FIGURE 5.
Subunit-specific active nuclear import of the TM3–4 loop. A, a schematic representation of the constructs used is shown. The fusion protein of GFP and β-galactosidase cannot pass the nuclear pore by diffusion due to its size (>150 kDa). Using a multiple cloning site between the proteins, the TM3–4 loops of different subunits or the basic motif 346RRKRR350 were inserted in frame. B, shown is confocal live fluorescence microscopy of HEK293 cells transfected with fusion proteins of GFP and β-galactosidase (β-gal-GFP, 153 kDa), the TM3–4 loop of hsGlyRα1 with GFP and β-galactosidase ((β-gal)-α1-GFP, 162 kDa), and the basic motif 346RRKRR350 with GFP and β-galactosidase ((β-gal)-RRKRR-GFP, 153 kDa). Although (β-gal)-GFP is excluded from the nucleus, (β-gal)-α1-GFP is actively imported into the nucleus. The isolated basic motif RRKRR, however, is not sufficient to mediate nuclear import. C, shown is confocal live fluorescence microscopy of HEK293 cells transfected with fusion proteins of GFP, β-galactosidase, and the TM3–4 loops of human GlyRα2 ((β-gal)-α2-GFP), GlyRα3K ((β-gal)-α3K-GFP), GlyRα3L ((β-gal)-α3L-GFP), and GlyRβ ((β-gal)-β-GFP). Fusion proteins (β-gal)-α1-GFP, (β-gal)-α3L-GFP, and (β-gal)-α3K-GFP, but not (β-gal)-α2-GFP and (β-gal)-β-GFP, are actively imported into the nucleus.