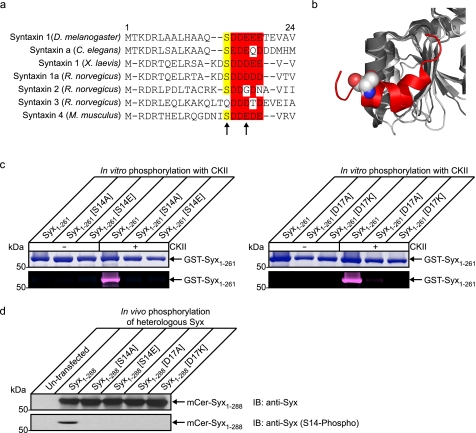
FIGURE 1.
Serine 14 is phosphorylated by CKII and is a key regulator of interaction with Munc18-1 at the plasma membrane. a, sequence alignment of syntaxin 1 orthologs and paralogs. The casein kinase II consensus site is highly conserved throughout evolution but is less conserved between mammalian paralogs. This may be indicative of a divergence of function between syntaxin isoforms. b, structural alignment of Munc18-1 (dark gray; Protein Data Bank code 3C98) (16) and Munc18-3 (light gray; Protein Data Bank code 2PJX) (15) of the region involved in mode 2/3 interaction with syntaxin. This region exhibits a very high degree of structural conservation indicative of an important functional role. Shown in red is the N-terminal peptide of syntaxin 4 bound to Munc18-3 with the serine residue highlighted in a space fill representation. c, to confirm that Ser14 is a casein kinase II phosphorylation site in syntaxin 1, multiple syntaxins were expressed and purified from bacteria (GST-Syx1–261). These were subjected to in vitro phosphorylation with detection using a fluorescent in-gel phosphostain. Total protein was observed by Coomassie staining. The cytoplasmic domain of syntaxin was readily phosphorylated by CKII in vitro, and this was blocked by mutation of Ser14 or the acidic consensus site at Asp17. d, to test these mutations in a cellular context, phosphorylation of heterologously expressed proteins was detected by immunoblotting (IB) using a Ser14 phospho-specific antibody. This demonstrated that heterologous syntaxin was phosphorylated on this site in vivo and was perturbed by modification of Ser14 or the CKII consensus site.