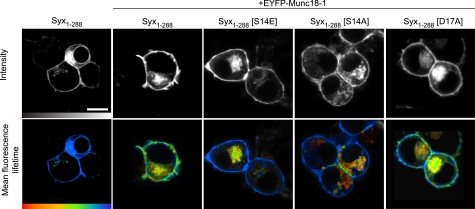
FIGURE 2.
Phosphorylation of syntaxin regulates its interaction with Munc18-1, specifically at the plasma membrane. TCSPC-FLIM reports FRET between mCerulean Syx1–288 (the donor) and EYFP-Munc18-1 (the acceptor) in live cells. Intensity images (upper row; gray scale) show wild-type and mutant mCerulean Syx1–288 localized to the plasma membrane of cells coexpressing EYFP-Munc18-1 (not shown in these images). TCSPC-FLIM data (bottom row) illustrate the excited state fluorescence lifetime of the donor. The mCerulean Syx1–288 exhibited a single fluorescence decay with a time constant of 2,288 ± 40 ps (mean ± S.E., n = 18) in the absence of an acceptor (EYFP and unfused Munc18-1). When coexpressed with EYFP-Munc18-1, this lifetime became significantly quenched (1,872 ± 333 ps, weighted mean of two components ± S.E., n = 5), indicative of FRET and protein interaction. The phosphomimetic form of syntaxin (Syx1–288(S14E)) returned the mean fluorescence lifetime to values not significantly different from that observed in the absence of an acceptor (2,035 ± 252 ps, weighted mean of two components ± S.E., n = 5). Notably, this change was restricted to the plasma membrane. The phospho-null syntaxin (Syx1–288(S14A)) resulted in a fluorescence lifetime not dissimilar to that observed for Syx1–288(S14E). The phospho-null syntaxin (Syx1–288(D17A)) had no detectable effect on the interaction. Scale bar, 5 μm. Color scale bar, 1,250–2,250 ps.