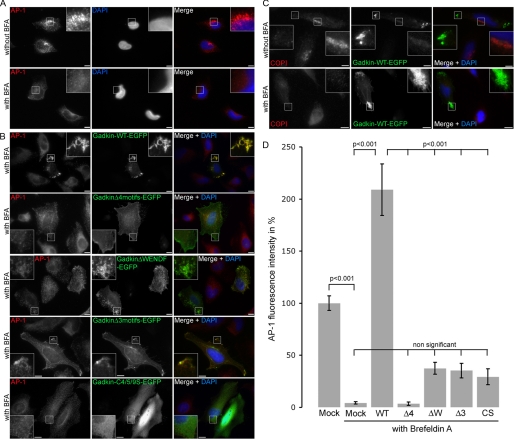
FIGURE 7.
The ability of Gadkin to prevent BFA-mediated dissociation of AP-1 from TGN/endosomal membranes depends on its S-palmitoylation and on direct binding to γ-ear. Gadkin mutants lacking the ability to bind to AP-1γ-ear (Δ4 motifs) or to undergo S-palmitoylation are unable to protect AP-1 from BFA-mediated membrane dissociation. A, HeLa cells were incubated without (top) or with (bottom) BFA and processed for indirect immunofluorescence microscopy using antibodies against AP-1. DAPI-stained nuclei are shown in blue. Scale bar, 10 μm. B, HeLa cells overexpressing Gadkin-eGFP WT or the indicated mutants were treated with BFA and processed for indirect immunofluorescence microscopy using antibodies against AP-1. DAPI-stained nuclei are shown in blue. Scale bar, 10 μm. C, HeLa cells overexpressing Gadkin-eGFP WT were treated with BFA and processed for indirect immunofluorescence microscopy using antibodies against COPI. DAPI-stained nuclei are shown in blue. Scale bar, 10 μm. D, quantification of the degree to which Gadkin WT or mutants (Δ4motifs, ΔWENDF, Δ3motifs, and C4/5/9S) protect AP-1 from BFA-induced membrane dissociation. AP-1 fluorescence intensity per cell was determined using Slidebook software and normalized to the intensity found in untransfected non-BFA treated cells. 36–76 cells were analyzed per sample. Data were pooled from two (for palmitoylation defective mutant) or four (all other mutants) independent experiments and given as mean values ± S.E. An analysis of variance multicomparison test, followed by a Tukey post-test, was used to assess statistical significance.