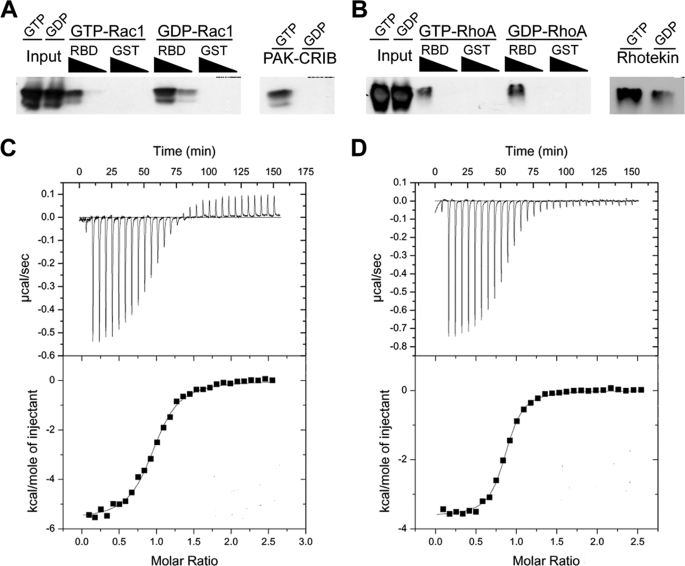
FIGURE 5.
YopO RBD shows no preference for Rac1 or RhoA in vitro. Purified recombinant Rac1 (A) and RhoA (B) were loaded with GDP or GTPγS and then incubated at 1 or 0.1 μm with 20 μg of GST-YopO RBD immobilized on glutathione-Sepharose beads. Following 1 h of incubation, eluates were analyzed by SDS-PAGE and Western blotting using antibodies to Rac and Rho. As a nucleotide loading control, GDP or GTPγS-bound Rac1 and RhoA were incubated with the Rac/Cdc42 binding domain of p21-activated kinase (PAK-CRIB) and the Rho binding domain of Rhotekin (Rhotekin) respectively, immobilized on glutathione-Sepharose beads, and analyzed as above. Interactions between purified G proteins and YopO RBD were further analyzed by isothermal titration calorimetry. Representative titrations of purified Rac1 (C) and RhoA (D) are shown. Experiments were repeated on at least two independent occasions with the same results.