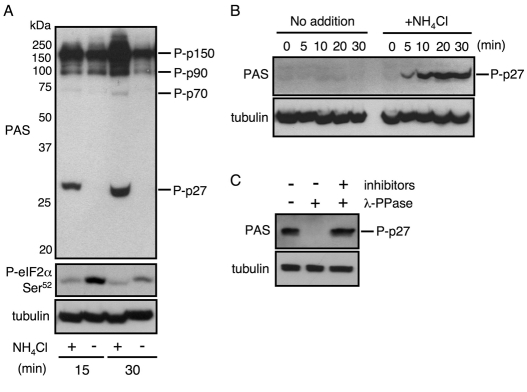
Fig. 1.
Phosphorylation of p27 in response to nitrogen availability is recognized by the anti-PAS antibody. (A) Cells of JUp1204 were cultured in EMM medium at 30°C to exponential phase, then washed and incubated in EMM with (+) or without (−) ammonium for the indicated time points. Cell extracts were probed with anti-PAS antibody or with anti-phospho-eIF2α (Ser51) antibody to detect phosphorylation of eIF2α at Ser52. In the top panel, positions of predicted phosphoproteins (p27, p70, p90 and p150) and molecular size markers are indicated. Tubulin is shown as a loading control (bottom). (B) Exponentially growing cells of JUp1204 were starved in EMM-N (a nitrogen-free version of EMM) for 1 hour, and then water (no addition) or 0.5% ammonium chloride (final concentration) was added. The cells were taken at the indicated time points, and extracts were probed with the anti-PAS antibody for phosphorylation of p27 or anti-tubulin antibody. (C) Extracts from exponentially growing cells of JUp1204 were treated with λ-phosphatase in the presence or absence of its inhibitors and probed for phosphorylation of p27 or tubulin.