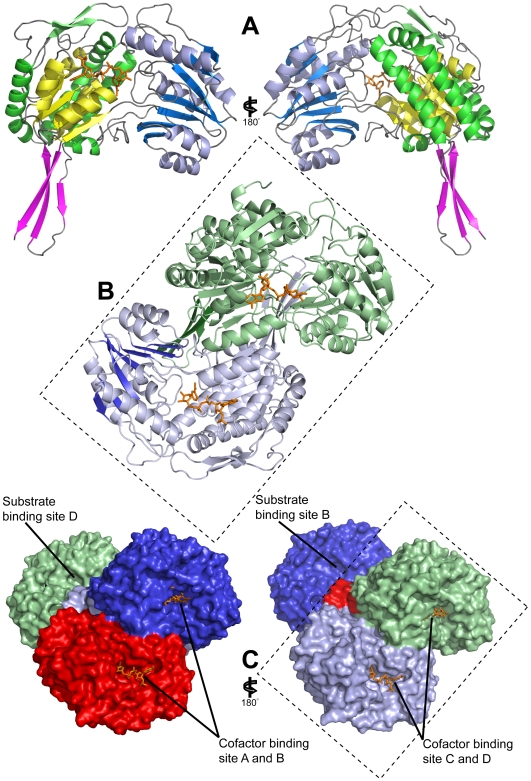
Figure 2. Crystal structure of E. coli SSADH.
a) Two cartoon representations of E. coli SSADH monomer (rotated by 180°) with NADP+ bound (orange) comprises of the catalytic domain (blue and light blue) with catalytic loop (red), the cofactor binding domain (green and yellow, where yellow illustrates the Rossmann fold) and the oligomerisation domain (magenta); b) A cartoon representation of the E. coli SSADH dimer with NADP+ bound (orange), it can be seen that the 3-stranded oligomerisation domain β- sheet (dark green) of the green monomer is extending the 7-stranded catalytic domain β- sheet (dark blue) of the blue monomer to form a 10-stranded β- sheet. c) Two surface representation models of the SSADH tetramer (rotated by 180°) showing the dimer of dimer formation between the blue and red monomer and the green and light blue monomers. NADP+ (orange) can be seen on the same face of the dimer, the substrate binding pocket has also been labelled.