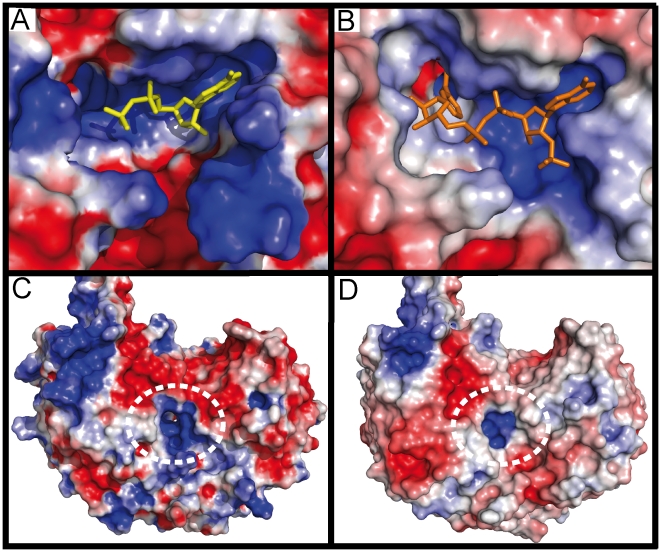
Figure 4. Comparisons between human (PDB ID: 2w8r)[26] and E.coli (monomer A) SSADH cofactor binding and SSA binding pockets, visualised using electrostatic surface representations (red represents negatively charged surfaces and blue represents positively charged surfaces).
A–B) both human (A: NAD+ in yellow) and E. coli (B: NADP+ in orange) SSADH have a two pocket NAD(P)+ binding site per molecule, the first (mostly blue), positively charged and close to the surface, accommodates the adenosine moiety (and the 2'phosphate in E. coli SSADH). The second binding pocket, deep in the active site, houses the nicotinamide ribose moiety (absent in human SSADH). The smaller human SSADH cofactor binding pocket has a large positive protrusion, which closes the bottom of the pocket, while the larger E. coli SSADH cofactor binding pocket can clearly accommodate the 2'phosphate of the NADP+. C–D) shows the positively charged SSA binding pocket of both human (C) and E. coli (D) SSADH highlighted by a white dashed line. The human SSA binding pocket is larger than the E. coli SSA binding pocket.