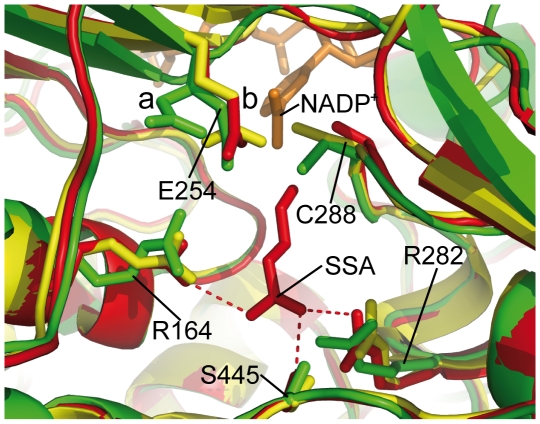
Figure 5. SSADH substrate binding and the active site.
A cartoon representation of the E. coli SSADH (monomer A: green) substrate (SSA) binding pocket superposed onto human SSADH C340A mutant with SSA bound (PDB ID: 2w8q [26]: red) and human SSADH containing the catalytic cysteine (PDB ID: 2w8o [26]: yellow). The key SSA binding residues from 2w8q have their interactions with SSA shown as a red dashed line. Superposition of catalytic residues are shown: the catalytic cysteine and the general base as sticks (labeled according the E. coli SSADH) and NADP+ (orange) from E. coli SSADH. It can be seen that the equivalent SSA binding residues from 2w8o and E. coli SSADH (R164, R283 and S445) are in a very similar location and orientation to those of 2w8q. The catalytic cysteine of 2w8o is oriented toward the NADP+ moiety while in E. coli C288 is oriented toward the substrate (SSA). Also two conformations of the general base (E254) can be seen in E. coli SSADH, with (a) being in the hydride conformation and (b) the hydrolysis conformation.