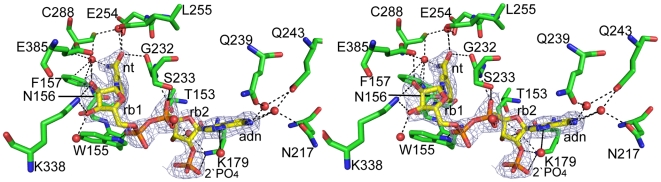
Figure 6. NADP+ binding of E. coli SSADH.
Stereo view of the active site showing the NADP+ moiety (yellow), SSADH residues (green) involved in binding NADP+, water molecules can be seen as red spheres and all bonds are depicted with a black dashed line. The 2F0–Fc omit electron density of the NADP+ moiety contoured at 1σ is also shown (light blue mesh). Interactions of monomer A and NADP+ can be seen, specifically both AN1 and AN6 of the adenine moiety (labelled adn) interacts with Q239(Oε1), Q243(Oε1) and N217(Oδ1) via water molecules. Adjacent to the adenine moiety, both AO2 and AO3 of the ribose (labelled rb2) hydrogen bonds with T153(O) and K179(NZ, O) via a single water molecule. 3AOP of the 2'-phosphate interacts with K179(NZ). AO2 of the pyrophosphate interacts with S233(N, OG) and the NO1 hydrogen bonds directly with W155(Nε1). Both NO2 and NO3 of the adjacent ribose moiety (labelled rb1) hydrogen bonds with K338(NZ), while NO2 also interacts with E385(Oε1). NN7 of the nicotinamide (labelled nt) moiety interacts with N156(Nδ2) and the catalytic C288(N) via the single water molecule. While NO7 interacts directly with G232(O) and L255(O), as well as with L255(N) and E254(Oε1) via the same water molecule. Up to 13 SSADH residues make 24 van der Waals or hydrogen bonds interactions with NADP+ per monomer, 16 of which are mediated by water (Table S2). Notably, all of the residues involved directly NADP+ binding in E. coli SSADH [48] (Table S2) are also conserved in human SSADH.