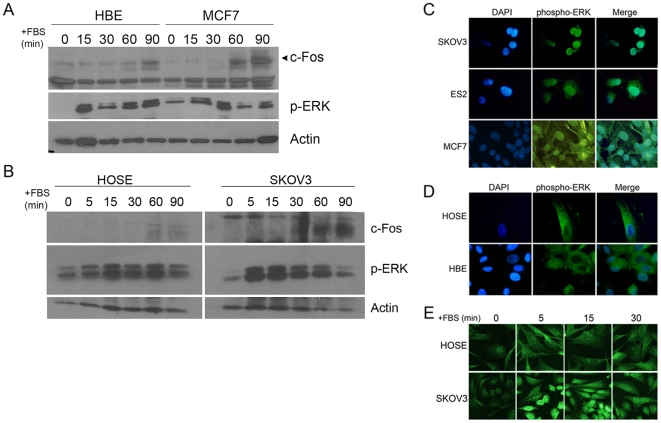
Figure 1. Nuclear localization of phospho-ERK is repressed in primary epithelial cells.
(A) Time course of ERK activation in HBE and MCF7 cells. Primary human breast epithelial (HBE) cells and MCF7 breast carcinoma cells were serum starved for 24 h to become quiescent (time 0), then stimulated with 15% FBS for 0–90 min, and phospho-ERK (p-ERK) and c-Fos expression, a downstream marker for nuclear phospho-ERK activity, were determined in cell lysates normalized for protein. (B) Time course of ERK and cFos activation in HOSE and SKOV3 cells. Primary human ovarian surface epithelial (HOSE) cells and SKOV3 ovarian carcinoma cells were grown as described for HBE and MCF7 cells, above. Expression of c-Fos and p-ERK was determined by immunoblotting. (C-E) Primary human breast and ovarian epithelial cells at passage 1 after isolation and breast and ovarian carcinoma cells were growth arrested in serum-free medium for 24 h, then stimulated with 15% serum, and analyzed for activated ERK (phospho-ERK). Cells for immunofluorescence were fixed and probed with an anti-phosphoERK1/2 monoclonal antibody (Sigma) followed by detection using a fluorescent secondary antibody. The nuclei were counterstained with DAPI and the two images merged using Adobe Photoshop, shown in the far right column. (C) Ovarian carcinoma SKOV3 and ES2 cells and MCF7 breast carcinoma cells show robust nuclear phospho-ERK localization after serum stimulation for 15 min. (D) In cultures of primary HOSE and HBE, phospho-ERK is localized primarily to the cytoplasm after serum stimulation for 15 min. (E) Primary cultures of early passage HOSE cells and SKOV3 were stimulated with FBS for the indicated times, then processed for immunofluorescence staining and confocal imaging. The results are representative of at least 3 experiments for each cell type.