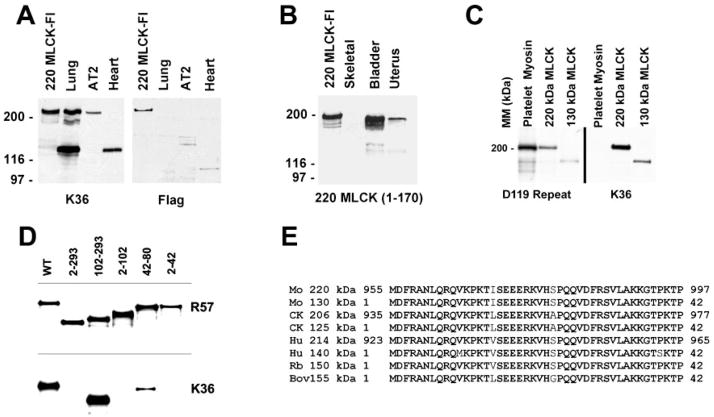
Fig. 1.
Expression and detection of 220-kDa myosin light chain kinase (MLCK). A: Western blot detecting expression of recombinant 220-kDa MLCK with the Flag epitope tag (220-kDa MLCK-Fl). Recombinant 220-kDa MLCK-Fl expressed in COS cells comigrates with 220-kDa MLCK expressed in lung and AT2 cardiomyocytes. The 220-kDa MLCK detected in COS cells represents both the recombinant 220-kDa MLCK-Fl and the endogenous 220-kDa MLCK and appears as a doublet on the original film of the Western blot reacted with the K36 monoclonal antibody. B: specificity of the 220MLCK1–170 antibody. Western blotting was used to characterize the specific reaction of an antibody to residues 1–107 of the 220-kDa MLCK with recombinant 220-kDa MLCK expressed in cells and the endogenous 220-kDa MLCK present in adult mouse bladder and uterine tissues. This antibody does not detect 130-kDa MLCK present in bladder and uterus. The lower-molecular-mass bands in some samples represent proteolytic degradation products. C: examination of the specificity of D119 and K36 antibodies for MLCK. Purified platelet myosin (NMHC-IIA) and COS cell extracts expressing either recombinant 220- or 130-kDa mouse MLCKs were analyzed by Western blotting to determine whether D119 repeat antibody and K36 antibodies specifically detect only MLCK. D119 cross-reacts with NMHC-IIA, and K36 is highly specific for MLCK. MM, molecular mass. D: mapping the epitope for monoclonal antibody K36. COS cells transiently expressing mutant 150-kDa rabbit MLCK (numbers indicate residues deleted) were examined to determine reactivity by Western blotting of the K36 monoclonal antibody. The smallest region analyzed that is undetectable by K36 is a deletion of residues 2–42. WT, wild type. E: alignment of residues corresponding to those between 2 and 42 of the 150-kDa rabbit MLCK and all cloned 210- to 220-kDa and 130- to 150-kDa MLCKs. Mo, mouse; CK, chicken; Hu, human; Rb, rabbit; Bov, bovine.