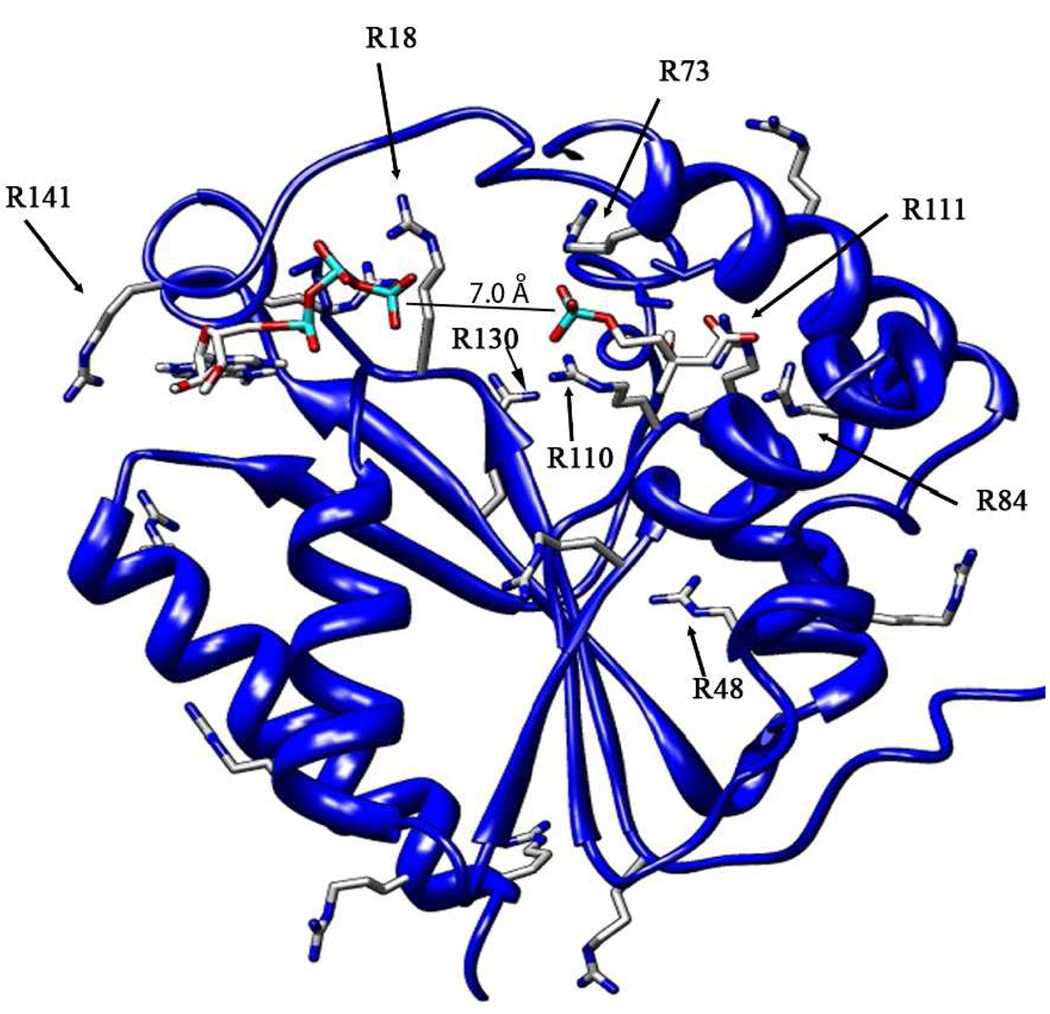
Figure 1.
Ternary complex of human PMK, with M5P and ATP docked independently into the apo crystal structure (3CH4).9 After docking, structure was optimized using molecular dynamics (50 ps at 300 K). All arginines are shown, and those known to be important for substrate binding or catalysis are labeled. Inter-phosphate distance is 7 Å, so phosphate transfer will require additional domain movement.