Abstract
The site(s) of interaction between human cytochrome P450 2B6 and NADPH-cytochrome P450 reductase (P450 reductase) have yet to be identified. To investigate this, the cross-linking agent 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) was used to covalently link P450 2B6 to P450 reductase. Following digestion with trypsin, the cross-linked peptides were identified by reconstituting the peptides in 18O-water based on the principle that the cross-linked peptides would be expected to incorporate twice as many 18O atoms as the non-cross-linked peptides. Subsequent mass spectrometric analyses of the resulting peptides led to the identification of one cross-linked peptide candidate. De novo sequencing of the peptide indicated that it is a complex between residues in the C-helix of the P450 (based upon solved x-ray crystal structures of P450 2B4) and the connecting domain of the P450 reductase. To confirm this experimentally, the P450 2B6 peptide identified through the cross-linking studies was synthesized and peptide competition studies were performed. In the presence of the synthetic peptide, P450 catalytic activity was decreased by up to 60% when compared to competition studies performed using a nonsense peptide. Taken together, these studies indicate that residues in the C-helix of P450 2B6 play a major role in the interaction with the P450 reductase.
Keywords: Cytochrome P450, crosslinking, protein-protein interactions
The cytochromes P450 (P450) enzymes belong to a family of heme containing proteins that catalyze the oxidative metabolism of a wide range of endogenous and exogenous substrates. All P450s share a common catalytic mechanism which involves the two-electron reduction of molecular oxygen resulting in the formation of a reactive oxygen intermediate and water [1]. Electrons are transferred from NADPH to the P450 via NADPH-cytochrome-P450 reductase (P450 reductase), which leads to the reductive activation of molecular oxygen followed by the insertion of one oxygen atom into the substrate [1].
In order to identify the P450 2B6 residues that may be involved in the interaction with P450 reductase, we used the water soluble carbodiimide, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) as a crosslinker. EDC has proven to be useful for the identification of sites of interaction between a number of protein-protein complexes including P450 reductase binding with cytochrome c and the binding of cytochrome b5 to P450 2E1 [2, 3]. EDC covalently links lysine residues to either aspartic or glutamic acid residues, making this an appropriate cross-linker to use in these studies since the interaction between the P450 and P450 reductase is believed to be electrostatic. The P450 2B6 and P450 reductase used in our studies were expressed and purified according to published protocols [4–6]. For cross-linking, full-length P450 2B6, P450 reductase and lipid (dilaurylphosphatidylcholine) were reconstituted using a molar ratio of 1:1:600, respectively. The P450 2B6 (200 pmol), P450 reductase (200 pmol) and lipid were incubated on ice for 45 min, and then allowed to sit at room temperature for 10 min. EDC (Pierce, Rockford, IL) was added to a final concentration of 10 mM from a 100 mM stock made fresh just prior to the experiment. After 2 h, the reaction was quenched by dialysis against 100 mM ammonium bicarbonate buffer, pH 8.5, twice for 4 h each. In order to identify the P450 2B6-P450 reductase cross-linked peptides we used 18O-water to isotopically label the cross-linked peptides following digestion by trypsin. Following proteolysis by an enzyme such as trypsin, oxygen atoms from water can specifically exchange with the two oxygen atoms of the carboxyl terminus of each tryptic peptide [7]. By comparing the masses of the peptides formed from the addition of either 18O-water or 16O-water following proteolysis, cross-linked peptides can be identified based on the principle that the incorporation of 18O atoms results in a mass shift greater than 4 Da. Isotopic labeling has been used previously to identify cross-linked peptides [2, 8, 9]. Therefore, trypsin was added to the EDC-treated samples following dialysis such that the amount of protein used in the EDC reaction was in 50-fold molar excess over the trypsin. The digestion was allowed to proceed for 24 h at 37°C. Subsequently, the samples were divided into two portions and dried down using a SpeedVac. The resulting peptides were then reconstituted using 50 µl of either 16O-water or 18O-water and analyzed using a LTQ mass spectrometer equipped with a photodiode array detector (Thermo Electron Finnigan) in the University of Michigan Biomedical Mass Spectrometry Facility. The peptides were resolved using a C18 reverse phase column (Luna, 3 µm, 4.6 × 100 mm, Phenomenex, Torrance, CA) using the following gradient: 20% B from 0–5 min; 20–70% B from 5–35 min; 70–90% B from 35–45 min at a flow rate of 0.2 mL/min. The solvent system consisted of solvent A (H2O, 0.1% formic acid) and solvent B (acetonitrile, 0.1% formic acid). The electrospray ionization (ESI) conditions were: sheath gas, 90 arbitrary units; auxiliary gas, 30 arbitrary units; spray voltage, 3.5 kV; capillary temperature, 200 °C; capillary voltage, 45 V; and tube lens offset, 25 V. Data were acquired in positive mode.
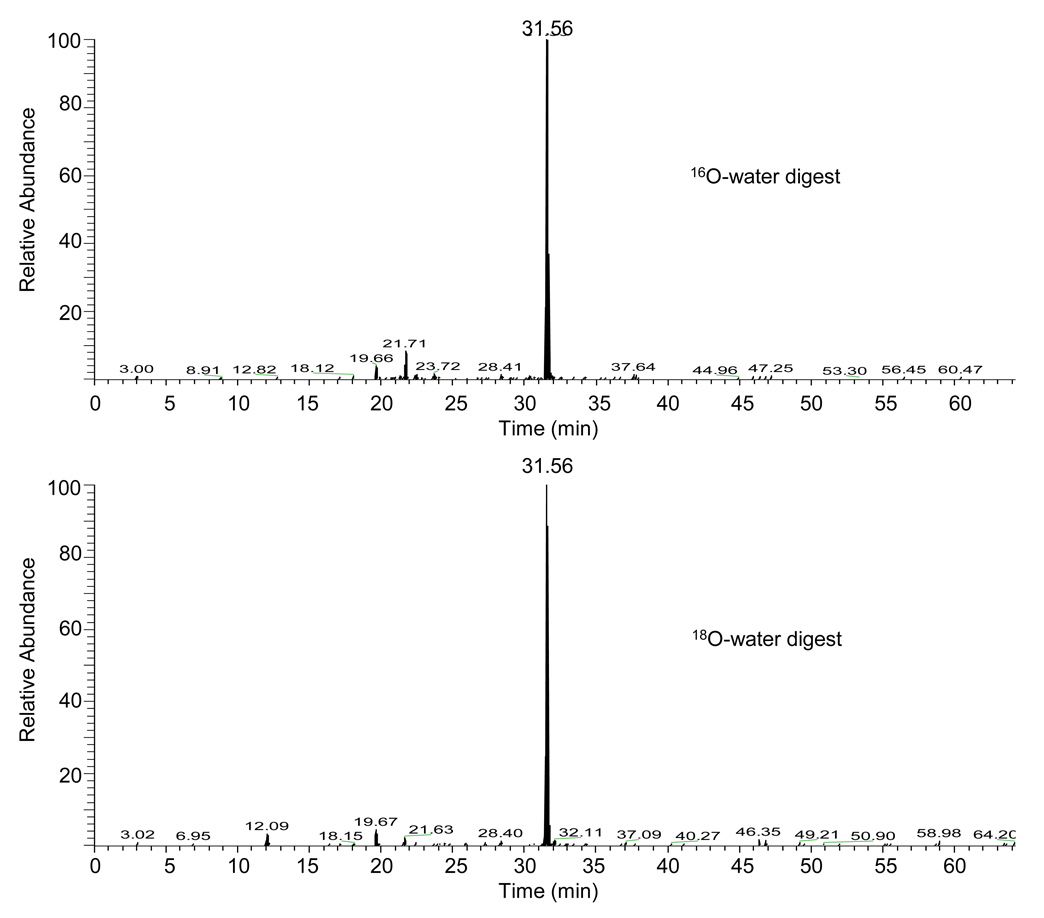
Under electrospray ionization conditions, peptide ions can exist in multiple charge states. In this case, the expected mass shift of the precursor ion is dependent upon the charge state of the ion. For instance, a singly charged ion upon the incorporation of four 18O atoms would have a mass shift of 8 Da when compared to the incorporation of four 16O atoms. However, a mass shift of 4 Da would be observed for a doubly charged ion. Through the use of the Pro-CrossLink software (obtained from the University of Washington Technology Transfer website) as well as manual analysis of the mass spectra, a cross-linked peptide candidate was identified with its quintuply charged ion at m/z 762.6 in the 16O-water samples and at m/z 763.8 in the 18O-water samples (Figure 1); these ions eluted with identical retention times and an ion at m/z 763.8 was not observed in the 16O-water sample. This mass shift of 1.2 for the quintuply charged ion indicates the incorporation of a total of three 18O atoms at the C-termini of this peptide. Using the Pro-CrossLink software, the cross-linked peptide (P450 2B6: DFGMGKR)-(P450 reductase: RHILAILQDCPSLRPPIDHLCELLPR) was identified based upon the mass and charge state of the monoisotopic peak at m/z 762.6, as well as the tandem mass spectrometry (MS/MS) spectrum of this ion. De novo sequencing of the peptide from the MS/MS spectrum shown in Figure 2 supports the identification of this peptide. This peptide was the only one for which the precursor ion detected in our studies exhibited the appropriate mass shift in the 18O sample and was abundant enough to generate MS/MS data.
Figure 1.
Extracted ion chromatograms for the elution of the quintuply charged ions with m/z values of 762.6 and m/z 763.8. The mass spectra of the cross-linked peptides were analyzed manually and using the Pro-CrossLink software to determine the mass shifts between the 16O-water and 18O-water reconstituted samples. The mass shift of 1.2 Da for the quintuply charged ion indicates the incorporation of three atoms of 18O at the C-terminus of this peptide. The ion with m/z 763.8 was not present in the 18O-water sample.
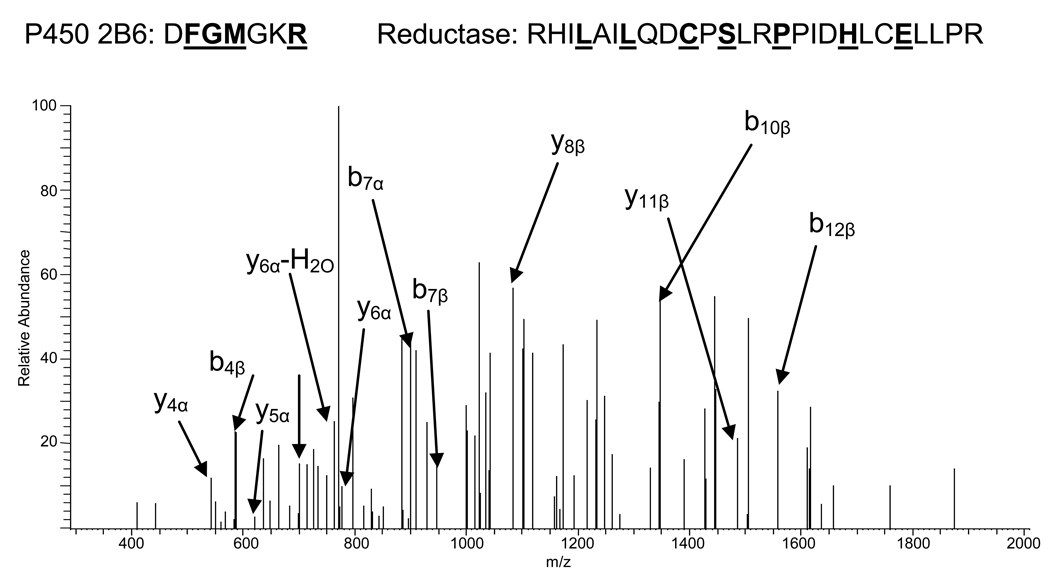
Figure 2.
MS/MS spectrum of the cross-linked precursor ion with [M+5]5+ = 762.0. The cross-linked candidate ion was fragmented for MS/MS using an LTQ mass spectrometer. De novo sequencing was used to determine the amino acid sequence of the peptide as shown above. The ions denoted as α refer to fragments from the P450 2B6 peptide D134-R140. The ions denoted as β are derived from the P450 reductase peptide R428-R453. Residues in underlined boldface type at the top of the figure represent amino acids identified in the spectrum.
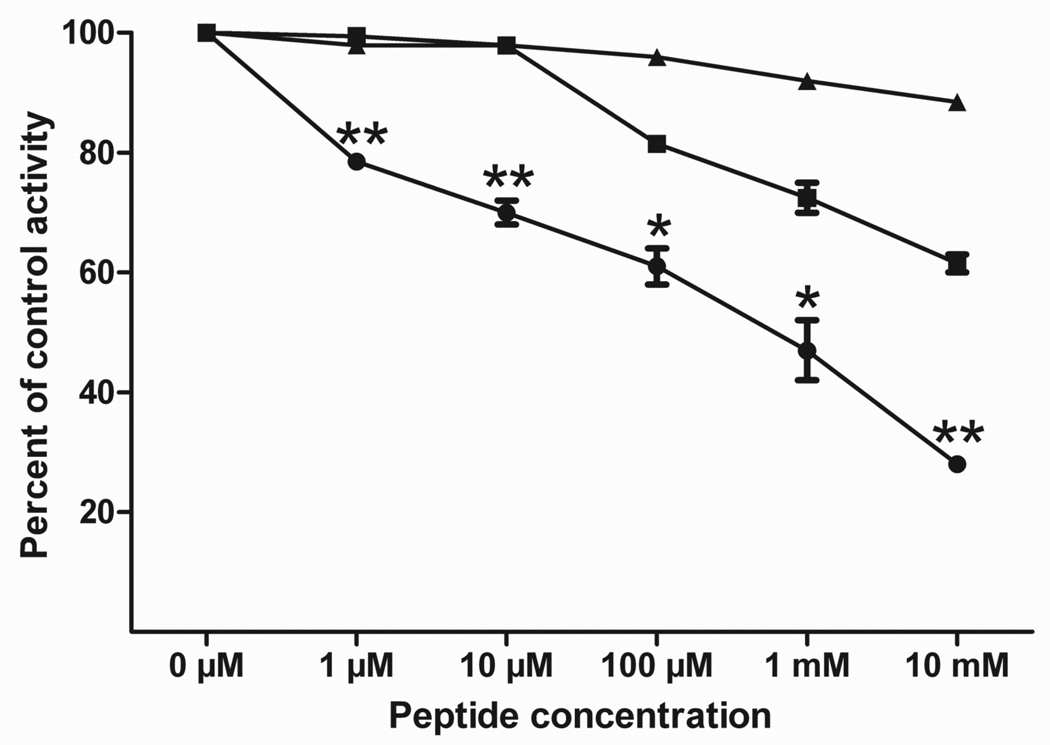
To confirm that the identified P450 2B6 peptide has the potential to play a role in the binding of the P450 to the P450 reductase, the synthetic peptides N-DFGMGKR-NH2 (the P450 peptide identified), H-GRMKGFD-NH2 (a peptide with those same amino acids scrambled) and H-VFCMGAR-NH2 (a nonsense peptide) were synthesized by ABGENT (San Diego, CA). P450 reductase (100 pmol) was incubated separately with each of the peptides (at concentrations of 0 µM, 1 µM, 10 µM, 100 µM, 1 mM and 10 mM) for 5 min at room temperature. P450 2B6 (100 pmol) was then added and incubated with the P450 reductase and peptide for 30 min at room temperature. Following the incubation, 100 mM KPi, pH 7.4, was added to bring the reaction volume to 100 µl. The substrate 7-ethoxy-4-(trifluoromethyl)coumarin (7-EFC) was added to a final concentration of 50 µM. The reaction was initiated using 2 mM NADPH and was allowed to proceed for 10 min at 30°C, and then quenched with 334 µL of acetonitrile. The amount of 7-hydroxy-4-(trifluoromethyl) coumarin formed was measured at room temperature using an excitation wavelength of 410 nm and an emission wavelength of 510 nm on a RF-5310 Spectrofluorophotometer (Shimadzu Scientific Instruments, Inc., Wood Dale, IL). As shown in Figure 3, P450 2B6 activity was decreased in a concentration-dependent manner in the presence of the synthetic DFGMGKR peptide, suggesting that the peptide was able to compete with P450 2B6 for P450 reductase binding. When compared to the nonsense peptide, the DFGMGKR peptide inhibited the P450 2B6 catalytic activity by up to 60%. The scrambled peptide exhibited some effect on the activity although significantly less than the DFGMGKR peptide. This is probably due to the fact that the peptide contains the same charged residues that are able to interact with P450 reductase in an electrostatic manner. Further, because the peptide is only seven amino acids in length, these residues are still in relatively close proximity to their locations in the DFGMGKR peptide.
Figure 3.
Inhibition of P450 2B6 activity by synthetic peptides. The incubations of the peptides with the P450 reductase and the subsequent determination of P450 2B6 activity were performed as described in the text. The peptides used were: (●) DFGMGKR, (■) Scrambled-GRMKGFD and (▲) Nonsense-VFCMGAR. The control activity refers to the activity observed in the absence of any synthetic peptide. Error bars represent the standard deviations. Data points representing incubation with the DFGMGKR peptide were statistically significant when compared to the corresponding scrambled-GRMKGFD peptide values. Data are labeled with one asterisk (*) if p<0.05 and two asterisks (**) if p<0.01. All of the DFGMGKR peptide data were statistically significant (p<0.01) when compared to the nonsense-VFCMGAR peptide. Significance was determined by performing one-tailed, unpaired t-tests using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, California).
The peptides we have identified in the present study include residues of P450 2B6 believed to be located in the C-helix (based upon the P450 2B4 crystal structure) [10, 11] and P450 reductase residues that lie in the connecting domain between the FAD and FMN domains. These same residues in the C-helix of P450 2B4 have been demonstrated by mutagenesis to be involved in the interaction between the P450 and P450 reductase [12]. The synthetic P450 2B6 peptide (H-DFGMGKR-NH2) used in our studies was able to compete for P450 reductase binding, as determined by measuring enzymatic activity; strongly suggesting that the amino acid residues that we identified using cross-linking and mass spectrometry are important to the P450 2B6-P450 reductase interaction. Co-crystallization of a P450 and P450 reductase may ultimately provide the most insight into the sites of interaction.
In summary, we have identified a P450 2B6-P450 reductase cross-linked peptide using mass spectrometry coupled with isotopic labeling. This study provides the first direct information regarding the sites of interaction between P450 2B6 and the P450 reductase.
Acknowledgements
This study was supported in part by grant CA16954 from the National Cancer Institute, National Institutes of Health (to P.F.H.), National Institutes of Health grant T32-GM007767 and a predoctoral fellowship in pharmacology/toxicology from the PhRMA Foundation (to N.N.B.).
Abbreviations
- P450
cytochrome P450
- P450 reductase
NADPH-cytochrome P450 reductase
- EDC
1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride
- ESI
electrospray ionization
- MS/MS
tandem mass spectrometry
- 7-EFC
7-ethoxy-4-(trifluoromethyl)coumarin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Isotopic labeling of cross-linked peptides coupled with mass spectrometry was used to identify P450 2B6 residues that potentially interact with NADPH-cytochrome P450 reductase. The identified amino acids lie in what is predicted to be the C/D loop of P450 2B6 based on the x-ray crystal structure of P450 2B4.
References
- 1.Porter TD, Coon MJ. J. Biol. Chem. 1991;266:13469–13472. [PubMed] [Google Scholar]
- 2.Gao Q, Doneanu CE, Shaffer SA, Adman ET, Goodlett DR, Nelson SD. J. Biol. Chem. 2006;281:20404–20417. doi: 10.1074/jbc.M601785200. [DOI] [PubMed] [Google Scholar]
- 3.Nisimoto Y. J. Biol. Chem. 1986;261:14232–14239. [PubMed] [Google Scholar]
- 4.Hanna IH, Reed JR, Guengerich FP, Hollenberg PF. Arch. Biochem. Biophys. 2000;376:206–216. doi: 10.1006/abbi.2000.1708. [DOI] [PubMed] [Google Scholar]
- 5.Hanna IH, Roberts ES, Hollenberg PF. Biochemistry. 1998;37:311–318. doi: 10.1021/bi971528s. [DOI] [PubMed] [Google Scholar]
- 6.Scott EE, Spatzenegger M, Halpert JR. Arch. Biochem. Biophys. 2001;395:57–68. doi: 10.1006/abbi.2001.2574. [DOI] [PubMed] [Google Scholar]
- 7.Fenselau C, Yao X. Methods Mol. Biol. 2007;359:135–142. doi: 10.1007/978-1-59745-255-7_9. [DOI] [PubMed] [Google Scholar]
- 8.Back JW, Notenboom V, de Koning LJ, Muijsers AO, Sixma TK, de Koster CG, de Jong L. Anal. Chem. 2002;74:4417–4422. doi: 10.1021/ac0257492. [DOI] [PubMed] [Google Scholar]
- 9.Collins CJ, Schilling B, Young M, Dollinger G, Guy RK. Bioorg. Med. Chem. Lett. 2003;13:4023–4026. doi: 10.1016/j.bmcl.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 10.Scott EE, He YA, Wester MR, White MA, Chin CC, Halpert JR, Johnson EF, Stout CD. Proc. Natl. Acad. Sci. U S A. 2003;100:13196–13201. doi: 10.1073/pnas.2133986100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott EE, White MA, He YA, Johnson EF, Stout CD, Halpert JR. J. Biol. Chem. 2004;279:27294–27301. doi: 10.1074/jbc.M403349200. [DOI] [PubMed] [Google Scholar]
- 12.Bridges A, Gruenke L, Chang YT, Vakser IA, Loew G, Waskell L. J. Biol. Chem. 1998;273:17036–17049. doi: 10.1074/jbc.273.27.17036. [DOI] [PubMed] [Google Scholar]