There was a time not so long ago when geneticists might have branded the highly migratory Atlantic bluefin tuna (Thunnus thynnus) the quintessential panmictic population (1) of the marine world. Because bluefin tuna are designed for mobility and capable of generating millions of offspring per spawning pair (2), migration and gene flow throughout their entire range seemed likely. This would homogenize regional subgroups and cause them to behave, in a statistical sense, like one very large, randomly mating population. In other words, bluefin appeared to fit the null hypothesis that population geneticists make a living trying to reject. Hence, genetic discontinuities should have been hard to find, especially within a small inland sea. In a study in PNAS, Riccioni et al. (3) examine microsatellite loci from contemporary and historical samples of T. thynnus and find remarkable evidence (i) that the Mediterranean contains genetically subdivided populations, (ii) that this structure has persisted for nearly 100 years, and (iii) that gene diversity has remained surprisingly intact despite decades of overexploitation and dramatic decline in numbers. These results confirm previous work (4, 5) hinting that T. thynnus in the Mediterranean is composed of multiple breeding stocks and raises concern that these newly recognized gene pools are at risk from overfishing.
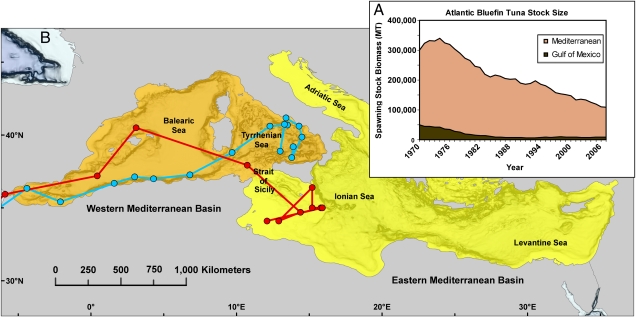
Atlantic bluefin tuna are migratory giants. Weighing upwards of 600 kg, they traverse the North Atlantic and adjacent seas between feeding and spawning grounds over a 30-year life span (6). Despite great historical abundance, spawning biomass (Fig. 1A) has plummeted over the last four decades (2). The International Convention for the Conservation of Atlantic Tunas, which coordinates management and research of highly migratory tunas, recognizes two populations (Western and Eastern Atlantic stocks) that spawn in the Gulf of Mexico and the Mediterranean Sea and manages them along a line bisecting the mid-Atlantic at the 45th meridian (6). Recently, the nation of Monaco requested that T. thynnus be listed under appendix I of the Convention on International Trade of Endangered Species, citing the alarming drop in the Mediterranean stock by 60% in the past 10 years (10).
Fig. 1.
(A) Decline over time of Atlantic bluefin tuna biomass in the Mediterranean- and Gulf of Mexico-spawning stocks (2). MT, metric tons. (B) Map of the Mediterranean Sea divided into eastern (yellow) and western (orange) basins with relevant subseas labeled (7). Blue (8) and red (9) lines are tracks of T. thynnus migrating into the Mediterranean Sea carrying electronic tags deployed off North Carolina.
Marine fish species had once been considered resilient to overexploitation, yet today marine fishery stocks worldwide are in precipitous decline (11). Under a panmictic model, one might assume that the depletion of one local population would be offset by the regular immigration or ongoing larval recruitment from another, both of which are enhanced by high fecundity. This has not always been the case and indicates that population structure could exist. However, analyses of neutrally evolving markers (mitochondrial DNA or microsatellites) have often failed to reject the null hypothesis, leaving behind conclusions that marine fish had little population structure to manage (12) or that genetic techniques failed to resolve the structure that was there (13).
Riccioni et al. note little loss of gene diversity in the Mediterranean.
To complicate matters more, evidence of genetic differentiation was often suspect and considered an artifact of high fecundity. Great reproductive success of only a few individuals could cause genetic variance (F ST’s) to fluctuate widely each generation (commonly called “genetic sweepstakes”) (14) such that differences one year should not be expected the next. In the study in PNAS (3), the authors include historical samples from juveniles and adults—insightfully preserved by Masimo Sella in the early part of the last century—to confirm that the differences that they find in Atlantic bluefin tuna inhabiting the Mediterranean Sea are temporally stable, and not a variable artifact.
Riccioni et al. (3) show that spatial differences between Atlantic bluefin sampled in the Adriatic and Tyrrhenian seas have persisted for nearly a century. Genetic differences between these two regions have been reported in other species (15), including red mullet (Mullus barbatus,), anchovies (Engraulis encrasicolus), and striped sea bream (Lithognathus mormyrus). Given the Atlantic bluefin’s reputation for wanderlust, it is odd that it would join a list of lesser vagile species with a similar geno-geographic pattern. However, the eastern and western basins of the Mediterranean differ in temperature, salinity, and circulation. Oceanographically speaking, these basins are considered partially isolated along the straits of Sicily and Messina (7) as shown in Fig. 1B.
Bluefin are known to spawn in the Balearic and Tyrrhenian seas on the Mediterranean’s western side and in the Ionian and possibly Levantine seas to the east (4, 5). Spawning in the Adriatic is unknown. Plotted onto Fig. 1B are migration tracks from two Atlantic bluefin (8, 9) tagged off North Carolina (roughly 7400 km away). After crossing the Atlantic Ocean, the bluefin pass through the Straits of Gibraltar where their tracks show remarkable salmon-like homing behavior to eastern and western Mediterranean spawning grounds located less than 500 km apart.
Fig. 1B illustrates the challenge in accurately assessing stock sizes of distinct bluefin populations. Eastern Mediterranean fish must cross western Mediterranean waters to reach their spawning grounds; if caught en route, they will be counted as part of the wrong population for stock assessments. The same issue confronts the western Atlantic (Gulf of Mexico-spawning) stock. A proportion of T. thynnus tagged off the coast of North Carolina belongs to the Mediterranean-spawning stock, and their presence in the western Atlantic catch can artificially inflate biomass estimates (16). One way to overcome this challenge is through the use of microsatellite markers. Multilocus genetic profiles characterizing each bluefin stock could make it possible to assign individuals to their population of origin. Such work could enable better accounting of migrating individuals captured in regions of population overlap as it has with salmonids (17).
Finally, despite of lower biomass, Riccioni et al. (3) note little loss of gene diversity in the Mediterranean. However, even in fish populations that have undergone more extensive declines than those documented here (e.g., Newfoundland cod, 75–99%), it is not always possible to observe bottlenecks in genetic data (18).
Atlantic bluefin tuna have long been a challenge for population geneticists. At first glance, they appear to be unproductive research choices. But a closer looks reveals a fine-scale structure. Although development of faster-evolving markers such as microsatellites has undoubtedly helped to resolve population structure in highly migratory fishes, better experimental design and elimination of mixed samples have played a more important role. Additional technologies (forensics, electronic tags, otolith microchemistry) combined with genetics will soon rewrite the dogma surrounding highly migratory marine fish and pave the way for geneticists to finally make significant contributions to the management and conservation of these fisheries. Decades ago, Sewall Wright (19) wrote, “There are species which appear to breed so nearly at random throughout their whole range…” But he added, “This is probably unusual.”
Acknowledgments
Electronic tag data for Fig. 1B were kindly provided by B. Block and plotted by M. Castleton. Genetic research of migratory marine fishery species is funded by the Lenfest Foundation, Monterey Bay Aquarium, and National Oceanic and Atmospheric Administration.
Footnotes
The author declares no conflict of interest.
See companion article on page 2102 in issue 5 of volume 107.
References
- 1.Wright S. Breeding structure of populations in relation to speciation. Am Nat. 1940;74:232–248. [Google Scholar]
- 2.International Commission for the Conservation of Atlantic Tunas . Report of the 2008 Atlantic Bluefin Stock Assessment Session (SCRS/2008/019) Madrid: International Commission for the Conservation of Atlantic Tunas; [Google Scholar]
- 3.Riccioni G, et al. Spatio-temporal population structuring and genetic diversity retention in depleted Atlantic bluefin tuna of the Mediterranean. Proc Natl Acad Sci USA. 2010;107:2102–2107. doi: 10.1073/pnas.0908281107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson J, et al. Microsatellite and mitochondrial DNA analyses of Atlantic bluefin tuna (Thunnus thynnus thynnus) population structure in the Mediterranean Sea. Mol Ecol. 2004;13:3345–3356. doi: 10.1111/j.1365-294X.2004.02336.x. [DOI] [PubMed] [Google Scholar]
- 5.Boustany AM, Reeb CA, Block BA. Mitochondrial DNA and electronic tracking reveal population structure of Atlantic bluefin tuna (Thunnus thynnus) Mar Biol. 2008;156:13–24. [Google Scholar]
- 6.National Research Council . An Assessment of Atlantic Bluefin Tuna. Washington, DC: National Academies Press; 1994. [Google Scholar]
- 7.Robinson AR, Leslie WG, Theocharis A, Lascaratos A. Mediterranean Sea circulation. In: Steele JH, Turekian KK, Thorpe SA, editors. Encyclopedia of Ocean Sciences. London: Academic Press; 2001. pp. 1689–1706. [Google Scholar]
- 8.Block BA, et al. Electronic tagging and population structure of Atlantic bluefin tuna. Nature. 2005;434:1121–1127. doi: 10.1038/nature03463. [DOI] [PubMed] [Google Scholar]
- 9.Walli A, et al. Seasonal movements, aggregations and diving behavior of Atlantic bluefin tuna (Thunnus thynnus) revealed with archival tags. PLoS One. 2009;4:e6151. doi: 10.1371/journal.pone.0006151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silfvergrip A. 2009. Supplementary information to the draft proposal to CoP15 to include bluefin tuna Thunnus thynnus on Appendix I of CITES as proposed at Monaco 18 September 2009.
- 11.Reynolds JD, Dulvy NK, Goodwin NB, Hutchings JA. Biology of extinction risk in marine fishes. Proc R Soc Lond B Biol Sci. 2005;272:2337–2344. doi: 10.1098/rspb.2005.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avise JC. Phylogeography: The History and Formation of Species. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- 13.Ward RD, Grewe PM. Appraisal of molecular genetic techniques in fisheries. Rev Fish Biol Fish. 1994;4:300–325. [Google Scholar]
- 14.Waples RS. Separating the wheat from the chaff: Patterns of genetic differentiation in high gene flow species. J Hered. 1998;89:438–450. [Google Scholar]
- 15.Maggio T, Brutto SL, Garoia F, Tinti F, Arculeo M. Microsatellite analysis of red mullet Mullus barbatus (Perciformes, Mullidae) reveals the isolation of the Adriatic Basin in the Mediterranean Sea. ICES J Mar Sci. 2009;66:1883–1891. [Google Scholar]
- 16.Kurota H, et al. A sequential Bayesian methodology to estimate movement and exploitation rates using electronic and conventional tag data: Application to Atlantic bluefin tuna (Thunnus thynnus) Can J Fish Aquat Sci. 2009;66:321–342. [Google Scholar]
- 17.Tonteri A, Veselov AJ, Zubchenko AV, Lumme J, Primmer CR. Microsatellites reveal clear genetic boundaries among Atlantic salmon (Salmo salar) populations from the Barents and White seas, northwest Russia. Can J Fish Aquat Sci. 2009;66:717–735. [Google Scholar]
- 18.Ruzzante DE, Taggart CT, Doyle RW, Cook D. Stability in the historical pattern of genetic structure of Newfoundland cod (Gadus morhua) despite the catastrophic decline in population size from 1964 to 1994. Conserv Genet. 2001;2:257–269. [Google Scholar]
- 19.Wright S. Evolution and the Genetics of Populations. Chicago: University of Chicago Press; 1969. Vol. 2. The Theory of Gene Frequencies. [Google Scholar]