Fig. 1. F5 fraction from Sf9 cells inhibited the formation of the AhR/Arnt/DRE gel shift complex.
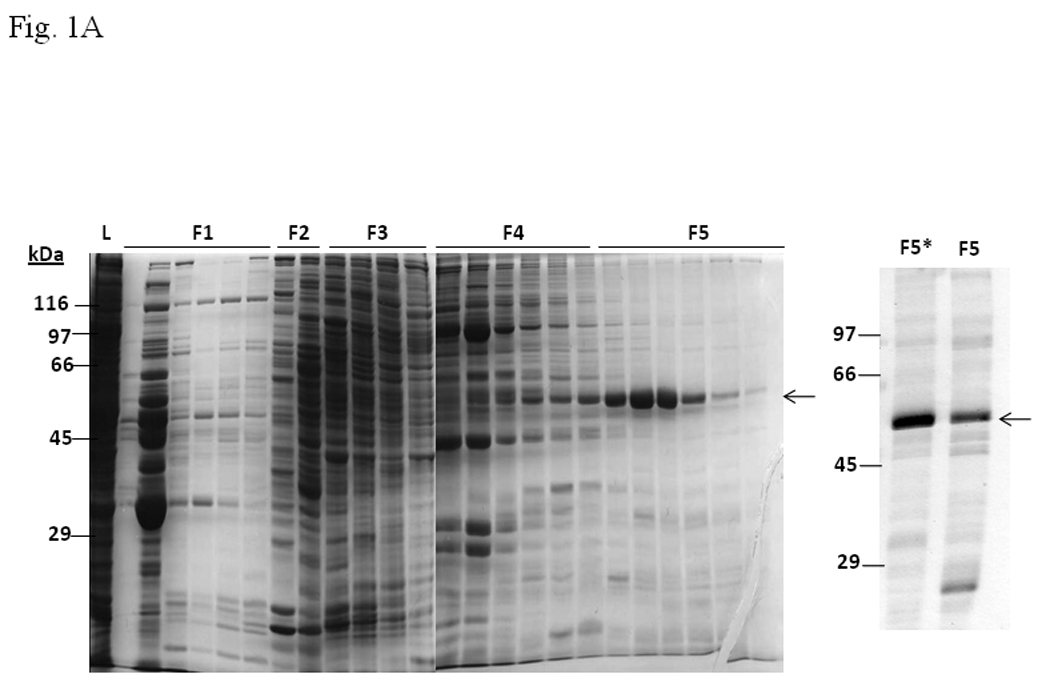

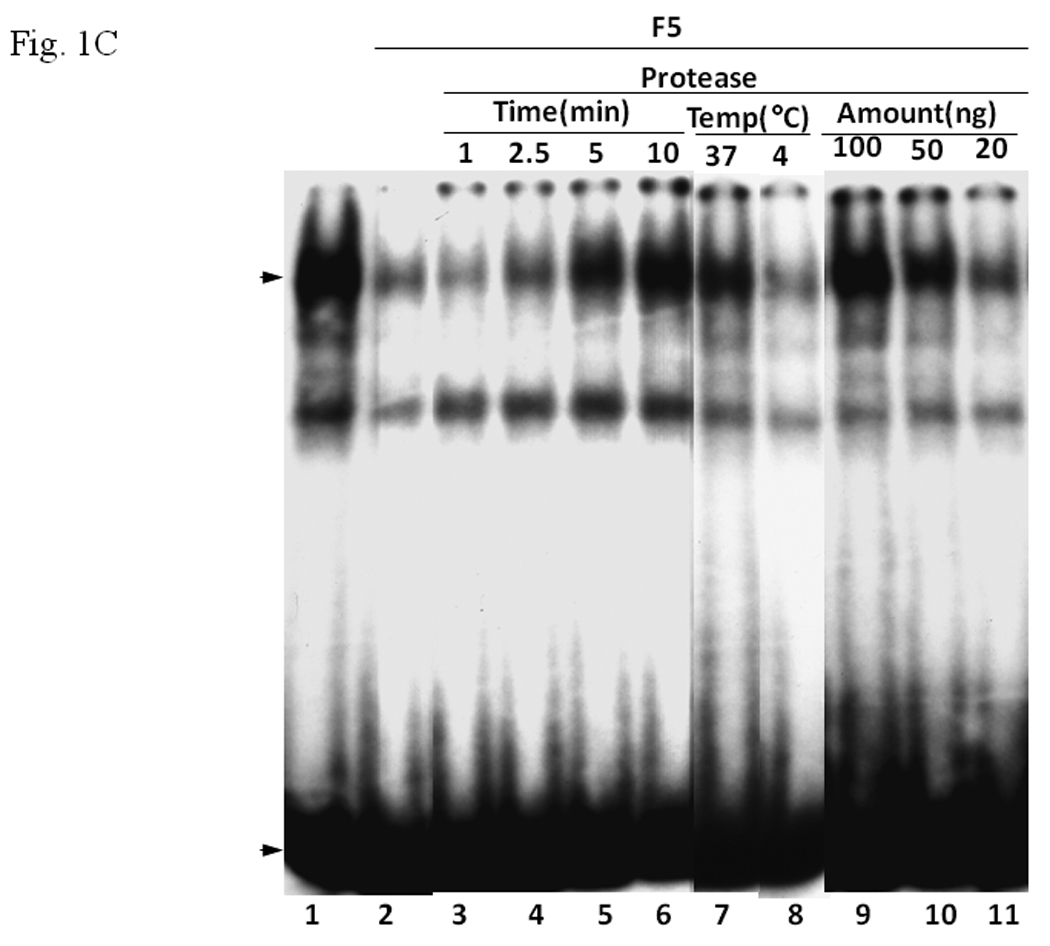
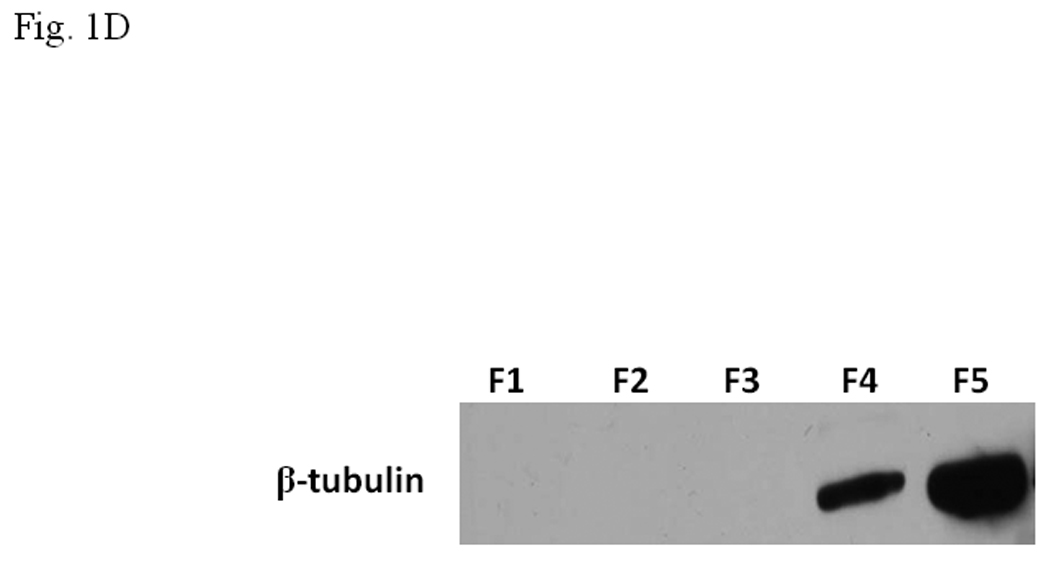
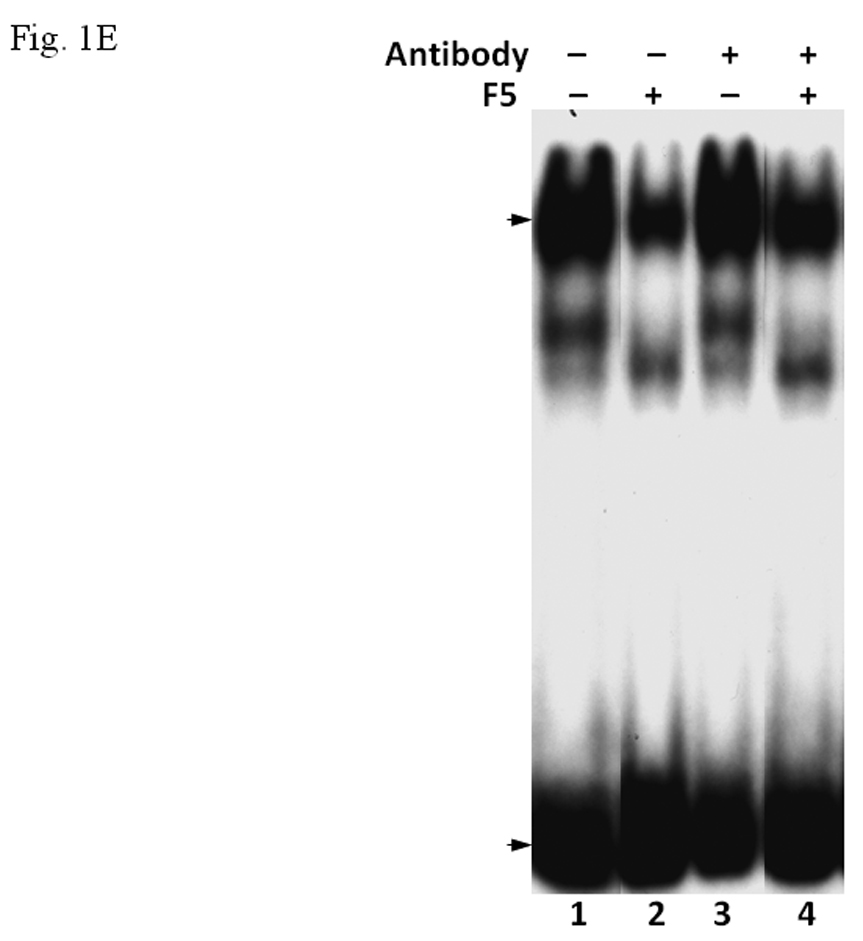
A. Coomassie blue staining of fractions from DEAE chromatography showing load (L) and fractions 1–5 (F1–F5). The arrow indicates the β-tubulin band. Right panel shows F5 and F5* (F5 subjected to gel filtration using FPLC to further enrich β-tubulin) of 5µg each. B. Gel shift assay showing the effect of F5 on the AhR/Arnt/DRE complex formation. All lanes contained baculovirus expressed human AhR, Arnt, Sf9 cytosol (2.5 µg) and 7 µM βNF. Concentration-dependent inhibition of the AhR/Arnt/DRE complex by F5 (lanes 2–4, 5–15 µg), BSA (lanes 5–7, 5–15 µg, negative controls) or F5 subjected to gel filtration to further enrich β-tubulin (F5*, lanes 9–11, 0.1–1 µg) was shown. Lanes 1 and 8 contained no F5, BSA or F5*. The upper arrow indicates the AhR/Arnt/DRE complex whereas the lower arrow indicates the free probe. Data are representative of two independent experiments. Lanes 8–11 contained 1µg of dIdC instead of the normal amount of 200 ng. C. Effect of protease (Sigma P4630, St. Louis, MO) treatment on F5 prior to gel shift assay. Protease inhibited the F5 effect in a time (lanes 3–6), temperature (lanes 7 and 8), and concentration (lanes 9–11) dependent manner. All lanes contained baculovirus expressed human AhR, Arnt, Sf9 cytosol (5 µg) and 7 µM βNF. Protease stock (1 µl) was used for each lane and the amount was listed in lanes 9–11. 100 ng of protease was used for lanes 3–8. The upper arrow indicates the AhR/Arnt/DRE complex whereas the lower arrow indicates the free probe. This gel shift assay was repeated twice with similar results. D. Western blot analysis of the F1–F5 fractions (10 µg) using anti-β-tubulin IgG T4026. E. Gel shift assay showing the effect of anti-β-tubulin IgG T4026 on the F5 inhibition of the AhR/Arnt/DRE complex. Gel shift assay showing that anti-β-tubulin IgG reversed the F5 inhibition (lanes 2 and 4) on the AhR gel shift complex. The upper arrow indicates the AhR/Arnt/DRE complex whereas the lower arrow indicates the free probe.
