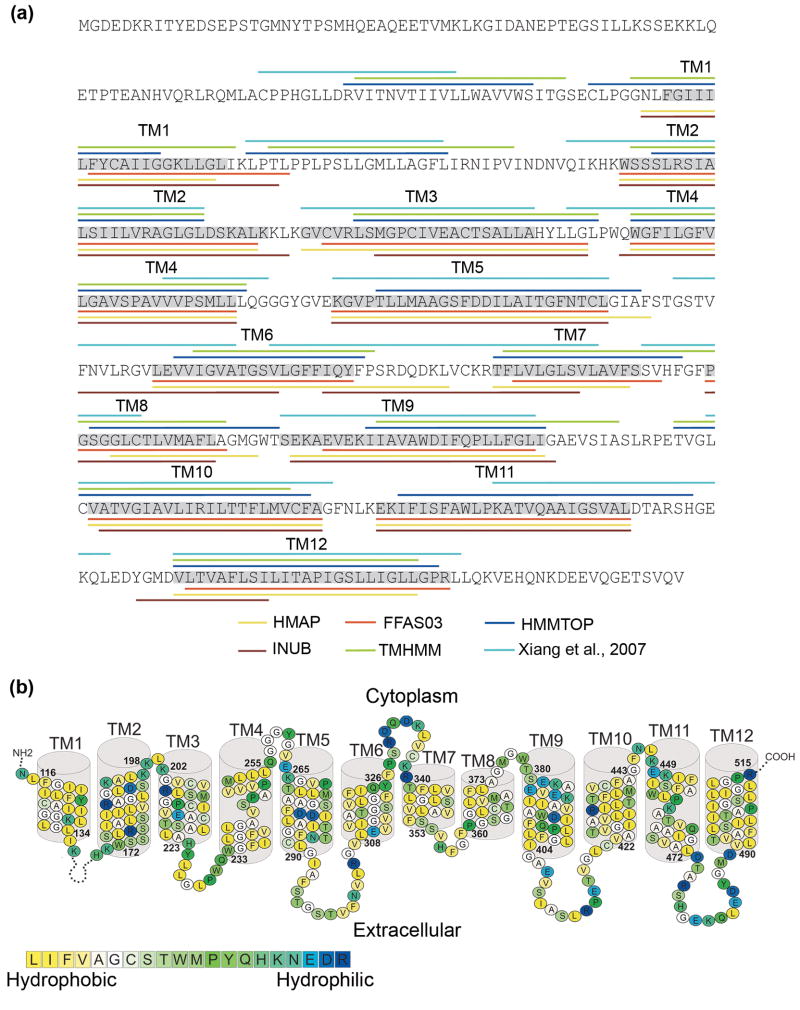
Fig. 1. Building the NHA2 model-structure.
(a) The location of the helices of NHA2 according to secondary structure predictions (HMMTOP and TMHMM), profile-to-profile alignment (HMAP), fold recognition (FFAS03 and INUB) and pairwise alignment with NhaA5 are marked in different colors according to the legends. The boundaries of the TM helices that were used for the modeling here are highlighted in grey, and the TM helix numbers are marked above. (b) The suggested TM topology of NHA2. Residues are colored according to the hydrophobicity scale of Kessel and Ben-Tal,30 using the color bar, with blue-through-yellow indicating hydrophilic-through-hydrophobic. The long loop connecting TM1 and TM2 was omitted for clarity. Overall, the helices are hydrophobic, but they do feature polar and titratable residues, as anticipated for a transporter.