Abstract
Early imaging or blood biomarkers of tumor response are desperately needed to customize antiangiogenic therapy for cancer patients. Anti–vascular endothelial growth factor (VEGF) therapy can “normalize” brain tumor vasculature by decreasing vessel diameter and permeability, and thinning the abnormally thick basement membrane. We hypothesized that the extent of vascular normalization will be predictive of outcome of anti-VEGF therapy in glioblastoma. We used advanced magnetic resonance imaging methods to monitor vascular parameters and treatment response in 31 recurrent glioblastoma patients enrolled in a phase II trial of cediranib, an oral pan-VEGF receptor tyrosine kinase inhibitor. We evaluated the correlation between clinical outcome and magnetic resonance imaging–measured changes in vascular permeability/flow (i.e., Ktrans) and in microvessel volume, and the change of circulating collagen IV levels, all after a single dose of cediranib. Here, we show that evaluation of biomarkers as early as after one day of anti-VEGF therapy with cediranib is predictive of response in patients with recurrent glioblastoma. Changes in Ktrans, microvessel volume, and circulating collagen IV correlated with duration of overall survival and/or progression-free survival (P < 0.05). When we combined these three parameters into a “vascular normalization index,” we found that it closely associated with overall survival (ρ = 0.54; P = 0.004) and progression-free survival (ρ = 0.6; P = 0.001). The vascular normalization index described here should be validated in randomized clinical trials.
Introduction
Several candidate biomarkers have been proposed for anti-vascular endothelial growth factor (VEGF) therapy with bevacizumab, an anti-VEGF antibody (Genentech). However, there are no validated predictive biomarkers for any anti-VEGF therapy in cancer (1). Identification of effective therapies in a timely fashion is critical for patients with limited survival, such as those with glioblastoma.
Functional magnetic resonance imaging (MRI) “water diffusion mapping” has been proposed as an early imaging biomarker for glioblastoma (2). However, glioblastomas secrete high VEGF levels, which lead to high vascular permeability and vasogenic edema (3, 4). Thus, blocking VEGF in glioblastoma likely leads to decreased vascular permeability, and edema alleviation (5, 6). Unfortunately, this could lead to difficulties in evaluating tumor response or diffusion maps using MRI (7). A recent study has identified a decrease in fluorothymidine uptake measured by positron emission tomography as a potential predictive marker for recurrent glioblastoma patients treated with bevacizumab and CPT-11 (8). Because the decrease in tumor uptake of fluorothymidine might also be confounded by a reduction in vascular permeability induced by bevacizumab, it is not clear if this biomarker reflects a decrease in permeability or proliferation.
Here, we combined imaging with circulating biomarkers. In preclinical models, anti-VEGF agents rapidly but transiently “normalize” the structure and function of glioblastoma vessels—they decrease vessel diameter and permeability, increase tumor oxygenation, and induce a thinning of the abnormally thick basement membrane (9, 10). Phase II trial data showed that cediranib (AstraZeneca, a pan-VEGF receptor tyrosine kinase inhibitor) decreased—as early as after 1 day of treatment—two neuroimaging biomarkers: vessel diameter (transiently) and permeability as assessed by Ktrans (more persistently) in a cohort of 16 consecutive recurrent glioblastoma patients (5). In further preclinical studies, we found that cediranib can improve survival in glioblastoma-bearing mice via edema reduction even in the face of persistent tumor growth (11). In that study, edema alleviation was due to transient normalization of the vasculature, and was associated with a transient increase in plasma collagen IV. We reasoned that increases in circulating collagen IV would reflect basement membrane thinning.
Because preclinical and clinical findings suggest that vascular normalization by cediranib therapy might be beneficial in glioblastoma patients, we hypothesized that changes at day 1 in three biomarkers related to vascular normalization would be predictive of improved survival in recurrent glioblastoma patients. Moreover, we hypothesized that a composite index based on the early changes in biomarkers of vascular normalization would more closely predict the outcome of cediranib treatment.
Materials and Methods
Dynamic Contrast-Enhanced–MRI Methodology
Image acquisition
All patients were scanned (with informed consent) at two baseline time points, typically 3 to 7 d (average, 5.7) and then 1 d before the first treatment, as well as 1 d after the first treatment on a 3 Tesla MRI system (Siemens), for the following key sequences:.
Dynamic contrast-enhanced images
This is a series of acquisitions of a 50.6-mm-thick slab consisting of 20 slices. All scans are 2.9 × 2.0 mm in-plane resolution, with a 2.1 mm slice thickness, 0.4 mm interslice gap, using a fast gradient echo technique (TR, 5.7 ms; TE, 2.73 ms). Data to allow computation of a T1 map of the tissue of interest are initially created using five different flip angles (2°, 5°, 10°, 15°, 30°). Then, the same slab of tissue is sampled with a 10° flip angle every 5.04 s for 252 s (50 time points), and 0.1 mMol/kg of gadopentetate-dimeglumine was injected 52 s after the beginning of the acquisition at 5 cc/s. Imaging time was 4 min and 12 s.
Permeability maps
Dynamic contrast-enhanced–MRI data were processed using custom made software written in Matlab to obtain maps of Ktrans (corresponding roughly to wash-in rates of the contrast agent; ref. 12). Ktrans can be influenced by flow, or by permeability, or both. In high-flow organs such as the brain, flow limitations are not usually a concern, but the blood-brain barrier severely limits permeability unless it is disrupted by disease. Even in such a state, Ktrans does not fully correspond to permeability, but it is related rather to the permeability-surface area product of the capillary bed (in nonflow-limited situations). Based on our previous data (5), Ktrans most likely is strongly related to permeability in this setting, although some flow dependence may be present as well.
Dynamic susceptibility contrast imaging
A 75-mm slab of tissue was imaged using a dual-echo, combined gradient-echo, and spin-echo planar sequence (TE, 34/103); each image had 1.7 mm in-plane resolution and 5 mm through plane resolution (128 × 128 matrix). There was a 2.5 mm interslice gap and 10 slices. We acquired 120 blocks of images—a block every 1.33 s. Gadopentetate-dimeglumine (0.2 mMol/kg) was injected at 5 cc/s after 85 s of imaging. Imaging time was 2 min and 45 s.
Blood volume maps
Relative CBV of smaller vessels (spin-echo images) was calculated using a standard deconvolution technique (13), with CBV corrected for leakage of the contrast agent across the blood-brain barrier (14, 15). These maps are relative and therefore unitless.
Patients continued cediranib monotherapy until progression, and we monitored the duration of PFS using T1-enhancement volume (Supplementary Figs. S1 and S2; Fig. 1).
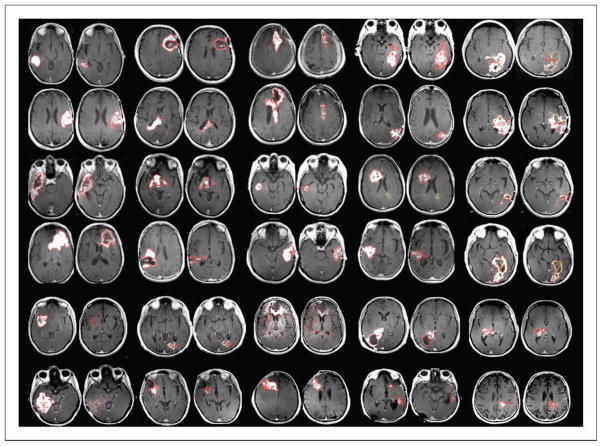
Figure 1.
Best responses after cediranib monotherapy in recurrent glioblastoma. Initial (left) and best-response (right; in each pair) postgadolinium axial T1-weighted MRI scans from 30 patients. Red outlines, the tumor borders as determined by expert neuroradiologists blinded to patient identity and scan date.
Measurement of Circulating Collagen IV
Peripheral blood was obtained with informed consent from all patients at baseline and 1 d following the first dose of cediranib. Blood was collected in an EDTA-containing vacutainer, spun down, and plasma was aliquoted, and frozen immediately. We measured the plasma levels of soluble collagen IV using ELISA kits from Exocell, Inc. We measured the plasma levels of Ang1/Ang2 using ELISA kits (R&D Systems) and matrix metalloproteinase 2 using MSD plates (MesoScale-Discovery). Samples were run in duplicate.
Statistical Analysis
We used multivariable Cox regression analyses of changes in three parameters: Ktrans, microvessel CBV, and plasma collagen IV 1 d after the first dose of cediranib. The vascular normalization index (VNI) was obtained as the negative of the linear predictor in the Cox regression with all three variables included, using regression’s coefficient estimates. For example, if coefficients in the Cox regression predicting hazard of death were a for Ktrans change (ΔKtrans), b for CBV change (ΔCBV), and c for plasma collagen IV changes (Δcoll IV), then the index was
We expressed the changes as the logarithm of the day 1 to pretreatment level ratios. We present hazard ratio estimates and P values from the Wald test. In addition, we used a recursive partitioning analysis classes—a prognostic factor of outcome used for stratification of glioblastoma patients in clinical studies in which age, extent of resection, performance, and mental status are combined into one discrete variable (16, 17). Furthermore, we adjusted for log-transformed pretreatment tumor volume. The importance of sequential addition to the regression model of Ktrans, collagen IV, and CBV was tested in the analysis of deviance table, using residual deviance statistic and related P values from the likelihood ratio test. (χ2 tests were performed to compare the observed improvement versus adding a random noise.) Associations between biomarker changes and progression-free survival (PFS; at 6 mo) and overall survival (OS; at 1 y) were analyzed using logistic regression with cubic-spline function of a covariate.
Results and Discussion
Cediranib efficacy in recurrent glioblastoma
Thirty of 31 patients enrolled had at least a transient radiographic response to cediranib (Fig. 1). One patient dropped out of the study before receiving cediranib. The median PFS (with 95% confidence intervals) of the 30 recurrent glioblastoma patients who received cediranib was 117 days (88–145; Supplementary Fig. S1). The median OS was 227 days (177–293; Supplementary Fig. S2). The rate of patients alive and progression-free at 6 months was 25.8% (14.7–47.9).
Association between biomarkers and outcome
Several of the parameters measured by us—functionally related to vascular normalization (5, 10, 11)—changed after 1 day of cediranib treatment (Supplementary Table S1). However, only the early changes in Ktrans, microvessel CBV, and plasma collagen IV correlated with survival outcomes (Supplementary Table S2; Fig. 2). Of the 30 patients treated, all three parameters were reliably measured in 28 patients.
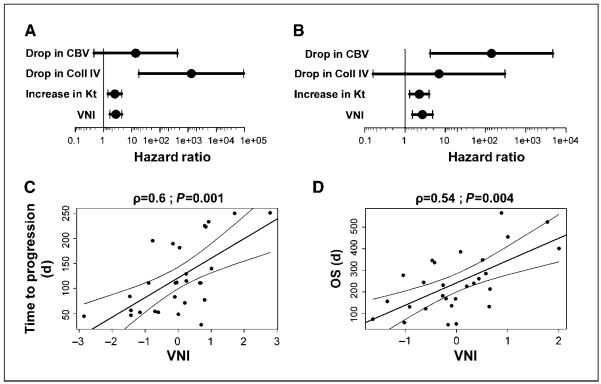
Figure 2.
Association between changes in Ktrans, CBV, and collagen IV and survival outcomes. A, correlations with PFS at 6 mo. B, correlations with OS at 1 y. C and D, scatter plots: VNI versus time-to-progression (C) and time-to-death (D).
A greater reduction in Ktrans after one dose of cediranib was seen in patients with increased PFS (P = 0.0015) and OS (P = 0.0039; Supplementary Fig. S3A and B). Of note, in two previous phase I studies of the pan-VEGF receptor tyrosine kinase inhibitor vatalanib (Novartis) in patients with colorectal cancer and metastatic liver lesions, favorable outcomes correlated with significantly greater reductions in Ki (a parameter related to Ktrans) at day 2 (18). Similarly, in advanced hepatocellular carcinoma patients, a greater reduction in Ktrans at 14 days after sunitinib (a VEGF receptor tyrosine kinase inhibitor; Pfizer) was significantly associated with increased PFS in a phase II study (19).
In addition, a greater increase in the CBV of tumor microvessels after one dose of cediranib was seen in the glioblastoma patients with extended OS (P = 0.0056; Supplementary Fig. S3C).
Finally, a greater increase in collagen IV levels in plasma was detected in patients with extended PFS (P = 0.0010; Supplementary Fig. S3D).
Association between a VNI and outcome
To test if we could more precisely predict outcomes after cediranib, we then combined these three parameters into a composite index. The index for prediction of OS was calculated as
and the index for prediction of PFS as
where x1, x2, and x3 denote log-transformed ratios of day 1 to baseline levels of, Ktrans, collagen IV, and CBV, respectively.
We found that this VNI closely correlated with PFS (Spearman’s ρ = 0.60; P = 0.001) and OS (ρ = 0.54; P = 0.004; Figs. 2C–D and 3). Analysis of residual deviance after sequential addition of variables into Cox regression showed that collagen IV is significant, in addition to Ktrans, for prediction of PFS (P = 0.002, likelihood ratio test); similarly, microvessel CBV is significant, in addition to Ktrans, for prediction of OS (P = 0.008; Supplementary Tables S3–5). Thus, collagen IV and microvessel CBV are important components of these regression models: the residual deviance reduction resulting from addition of both collagen IV and microvessel CBV is higher than the deviance reduction for Ktrans alone.
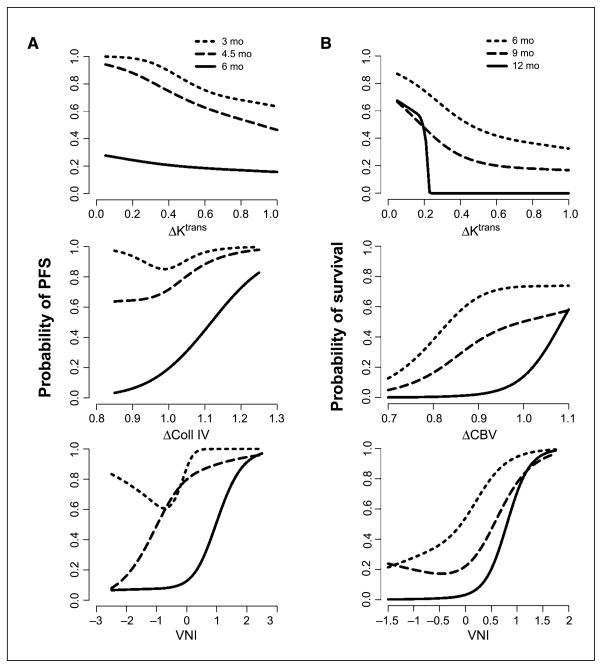
Figure 3.
Multivariate analyses for correlation between parameters related to vascular normalization and survival outcomes. A, the ratio of Ktrans and of collagen IV measured at day 1 and baseline and VNI values are significantly correlated with PFS at 3, 4.5, and 6 mo. B, the ratio of Ktrans and of microvessel CBV measured at day 1 and baseline and VNI values are significantly correlated with OS at 6, 9, and 12 mo. Strikingly, all 17 patients with Ktrans values at day 1 higher than 25% of baseline value died within 1 y, whereas 5 (42%) of the 12 patients with a Ktrans drop below 25% of baseline value survived >1 y.
Next, we stratified patients by score quartiles and found that Kaplan-Meier estimates of PFS and OS were gradually increasing according to the quartile group (Fig. 4). The VNI differentiated patients with favorable versus unfavorable outcome after a single dose of cediranib, indicating that evaluating the degree of tumor “vascular normalization” immediately after anti-VEGF treatment may be potentially used to predict better outcome in glioblastoma.
Figure 4.
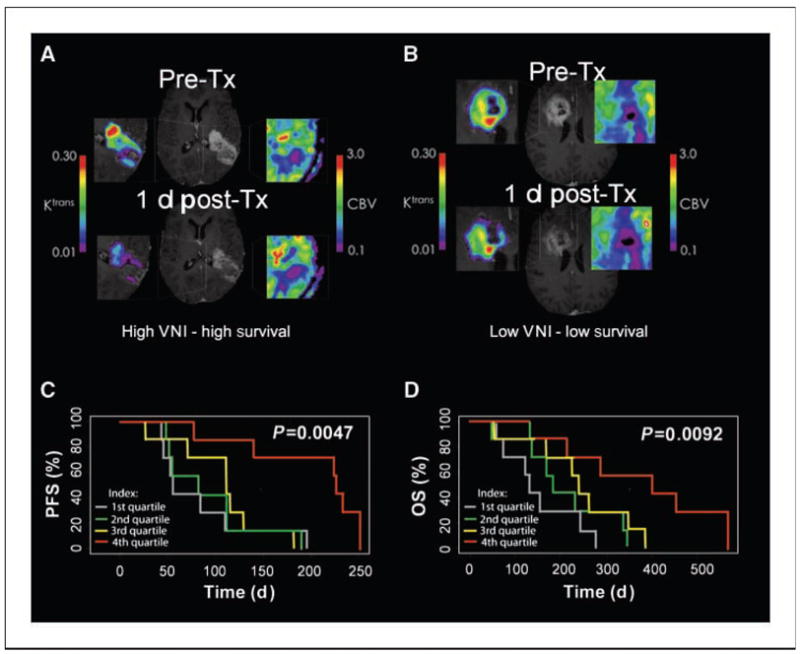
Potential predictive value of immediate changes in parameters related to vascular normalization after cediranib. A and B, representative patient examples: a patient with a high VNI and 566-d survival (A), versus a patient with a low VNI and 58-d survival (B); note especially the dramatic change in Ktrans, which decreased in both patients, albeit to differing degrees. Pre-Tx, pretreatment; post-Tx, postreatment. C and D, Kaplan-Meier PFS (C) and OS (D) distributions for index quartiles.
The prognostic recursive partitioning analysis class was 3 for 12 patients (40%), 4 for 12 patients (40%), and 5 for 6 (20%) of patients. Recursive partitioning analysis value showed only a trend for correlation with OS (P = 0.096), potentially due to the small sample size. In multivariate analysis, the associations between PFS and change at day 1 in Ktrans and circulating collagen IV, and VNI, and between OS and change at day 1 in Ktrans and CBV, and VNI were highly significant (Supplementary Fig. S4, Tables S3, S6, and S7; Fig. 3).
Implications
Although PFS evaluated by volumetric measurements of tumor enhancement correlated with OS in this study (Spearman’s ρ = 0.69), it cannot be used as a “predictive biomarker.” The ability to identify tumor-specific changes rapidly after treatment may allow tailoring of therapy to those patients most likely to benefit, and early discontinuation of an ineffective therapy in others. In addition, these biomarkers could also be valuable for treatment of benign tumors (e.g., schwannomas) or nonneoplastic diseases (e.g., macular degeneration) characterized by abnormal vessels (20). The biomarker candidates from this hypothesis-generating study need to be validated in large trials as predictive biomarkers and methodology needs to be established for its clinical use in individual glioblastoma patients.
Supplementary Material
Supplementary Figure S1: Kaplan-Meier estimate of the progression-free survival function after cediranib treatment in recurrent glioblastoma patients (n=31). The figures show regression lines with 95% predictive confidence intervals.
Supplementary Figure S2: Kaplan-Meier estimate of the overall survival function after cediranib treatment in recurrent glioblastoma patients (n=31). The figures show regression lines with 95% predictive confidence intervals.
Supplementary Figure S3: Univariate analyses for correlation between parameters related to vascular normalization and survival outcomes. The ratio of Ktrans values measured at day 1 and baseline are significantly correlated with progression-free survival (PFS) at 3, 4.5, and 6 months (a) and overall survival (OS) at 6, 9, and 12 months (b) after treatment onset. The extent of circulating collagen IV change at day 1 is significantly correlated with PFS at 3, 4.5, and 6 months (c), and the extent of microvessel CBV change at day 1 is significantly correlated with OS at 6, 9, and 12 months (d).
Supplementary Figure S4: Plots depicting association (hazard ratios with 95% confidence intervals obtained in a multivariable Cox regression) between changes in Ktrans, CBV, collagen IV, CE-T1 tumor enhancement volume and RPA (recursive partitioning analysis) class and survival outcomes. (a) Correlations with progression-free survival (PFS) at six months. (b) Correlations with overall survival OS at one year.
Supplementary Table S1: Kinetics of imaging and plasma biomarkers (pg/ml) – that are mechanistically related to tumor vascular normalization – after cediranib treatment in the recurrent GBM patients. Data are shown as medians and interquartile ranges (in square brackets) measured pre-treatment (Pre-Tx) and 1 day post-treatment.
Supplementary Table S2: Multivariable models of association between log-transformed changes at day 1 after cediranib treatment in Ktrans, collagen IV, and microvascular CBV with radiographic progression of disease and mortality in patients with recurrent GBM. Data are shown as hazard ratios with 95% confidence intervals (in square brackets).
Supplementary Table S3: Multivariable models of association between RPA class, log-transformed baseline CE-T1 volume, and changes at day 1 after cediranib treatment in Ktrans, collagen IV, and microvascular CBV with radiographic progression of disease and mortality in patients with recurrent GBM. Data are shown as hazard ratios with 95% confidence intervals (in square brackets).
Supplementary Table S4: The residual deviance table for six-month progression-free survival of disease for three parameters
Supplementary Table S5: The residual deviance table for overall survival for three parameters
Supplementary Table S6: The residual deviance table for six-month progression-free survival for five parameters
Supplementary Table S7: The residual deviance table for overall survival for five parameters
Acknowledgments
Grant support: NIH grants P01-CA80124, R01-CA115767, R21-CA117079, K24-CA125440, R01-CA129371, P41-RR014075, M01-RR-01066, and Federal Share/National Cancer Institute Proton Beam Program Income grants, Harvard Clinical and Translational Science Center, Montesi Family Research Fund, and Damon Runyon Foundation.
We thank G. Gorospe, C. Koppel, T. Benner, M. Foley, O. Wu, and S. Roberge for outstanding technical assistance.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
A.G. Sorensen: Commercial research grants, AstraZeneca, Exelixis, Genentech, Novartis, Schering-Plough, Siemens, and Takeda-Millennium; consultant/advisory board, EPIX Pharmaceuticals, AstraZeneca, Genentech, Takeda-Millennium, Bayer/Schering, Olea Medical, Mitsubishi, Novartis, and Siemens. T.T. Batchelor: Honoraria from speakers bureau, Vertex, Schering-Plough, and Enzon; consultant/advisory board, AstraZeneca, Genentech, and Takeda-Millennium. R.K. Jain: Commercial research grant, AstraZeneca and Dyax; consultant/advisory board, AstraZeneca, Dyax, Millennium, and SynDevRx. The other authors disclosed no potential conflicts of interest.
References
- 1.Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–38. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamstra DA, Galban CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol. 2008;26:3387–94. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–80. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 4.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–22. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 5.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen AG, Batchelor TT, Wen PY, Zhang WT, Jain RK. Response criteria for glioma. Nat Clin Pract Oncol. 2008;5:634–44. doi: 10.1038/ncponc1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol. 2007;25:4714–21. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 9.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 10.Winkler F, Kozin SV, Tong R, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1 and matrix metalloproteinases. Cancer Cell. 2004;6:553–63. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Kamoun WS, Ley CD, Farrar CT, et al. Edema control by cediranib, a VEGF targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27:2542–52. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–32. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Ostergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: Experimental comparison and preliminary results. Magn Reson Med. 1996;36:726–36. doi: 10.1002/mrm.1910360511. [DOI] [PubMed] [Google Scholar]
- 14.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27:859–67. [PMC free article] [PubMed] [Google Scholar]
- 15.Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology. 2002;222:715–21. doi: 10.1148/radiol.2223010558. [DOI] [PubMed] [Google Scholar]
- 16.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–10. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 17.Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–6. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan B, Thomas AL, Drevs J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003;21:3955–64. doi: 10.1200/JCO.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 19.Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. doi: 10.1200/JCO.2008.20.9908. Epub 2009 May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain RK. Taming vessels to treat cancer. Sci Am. 2008;298:56–63. doi: 10.1038/scientificamerican0108-56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Kaplan-Meier estimate of the progression-free survival function after cediranib treatment in recurrent glioblastoma patients (n=31). The figures show regression lines with 95% predictive confidence intervals.
Supplementary Figure S2: Kaplan-Meier estimate of the overall survival function after cediranib treatment in recurrent glioblastoma patients (n=31). The figures show regression lines with 95% predictive confidence intervals.
Supplementary Figure S3: Univariate analyses for correlation between parameters related to vascular normalization and survival outcomes. The ratio of Ktrans values measured at day 1 and baseline are significantly correlated with progression-free survival (PFS) at 3, 4.5, and 6 months (a) and overall survival (OS) at 6, 9, and 12 months (b) after treatment onset. The extent of circulating collagen IV change at day 1 is significantly correlated with PFS at 3, 4.5, and 6 months (c), and the extent of microvessel CBV change at day 1 is significantly correlated with OS at 6, 9, and 12 months (d).
Supplementary Figure S4: Plots depicting association (hazard ratios with 95% confidence intervals obtained in a multivariable Cox regression) between changes in Ktrans, CBV, collagen IV, CE-T1 tumor enhancement volume and RPA (recursive partitioning analysis) class and survival outcomes. (a) Correlations with progression-free survival (PFS) at six months. (b) Correlations with overall survival OS at one year.
Supplementary Table S1: Kinetics of imaging and plasma biomarkers (pg/ml) – that are mechanistically related to tumor vascular normalization – after cediranib treatment in the recurrent GBM patients. Data are shown as medians and interquartile ranges (in square brackets) measured pre-treatment (Pre-Tx) and 1 day post-treatment.
Supplementary Table S2: Multivariable models of association between log-transformed changes at day 1 after cediranib treatment in Ktrans, collagen IV, and microvascular CBV with radiographic progression of disease and mortality in patients with recurrent GBM. Data are shown as hazard ratios with 95% confidence intervals (in square brackets).
Supplementary Table S3: Multivariable models of association between RPA class, log-transformed baseline CE-T1 volume, and changes at day 1 after cediranib treatment in Ktrans, collagen IV, and microvascular CBV with radiographic progression of disease and mortality in patients with recurrent GBM. Data are shown as hazard ratios with 95% confidence intervals (in square brackets).
Supplementary Table S4: The residual deviance table for six-month progression-free survival of disease for three parameters
Supplementary Table S5: The residual deviance table for overall survival for three parameters
Supplementary Table S6: The residual deviance table for six-month progression-free survival for five parameters
Supplementary Table S7: The residual deviance table for overall survival for five parameters