Abstract
Osteonecrosis of the jaw (ONJ) has received significant attention as a potential side effect of bisphosphonate treatment. The limited understanding of the underlying pathophysiology of the condition emphasizes the need to transition ONJ research from the bedside to the bench, supplementing ongoing clinical research with animal/basic science studies. The goal of this review is to briefly highlight the most commonly proposed mechanisms for ONJ and then summarize our laboratory's recent efforts to begin transitioning ONJ research to an animal model. Remodeling suppression, disrupted angiogenesis and infection have all been proposed to connect bisphosphonates to ONJ, although most supportive data for each of these are either indirect or nonexistent. Our laboratory has begun studying the dog as a potential model of ONJ. We have shown regions of necrotic bone matrix within the mandible of dogs treated with oral or intravenous bisphosphonate. We hypothesize these regions are the result of remodeling suppression, and if combined with additional factors such as dental intervention or infection, would result in manifestation of exposed oral lesions, the clinical definition of ONJ. Although these findings suggest the dog may be a viable animal model to study ONJ, many questions remain unanswered. No matter what animal model is found to mimic the clinical presentation of ONJ, once established it will allow significant progress toward understanding the specific role of bisphosphonates in the pathophysiology of ONJ and if/how the entity of ONJ can best be treated and prevented.
Key Words: Animal models, Bisphosphonate, Osteonecrosis of the jaw, Mandible, Remodeling suppression, Review
Introduction
Since first brought to light in 2003 [Marx, 2003; Mehrotra et al., 2003], osteonecrosis of the jaw (ONJ) has received significant attention as a potential side effect of bisphosphonate treatment. PubMed currently lists over 300 citations pertaining to bisphosphonates and ONJ, all of which are case studies, reviews, editorials and position statements. Although these publications have been invaluable for documenting, summarizing and clarifying the prevalence, risk factors and management strategies for ONJ, they have provided limited data on the underlying pathophysiology of the condition. This emphasizes the need to transition ONJ research from the bedside to the bench, supplementing ongoing clinical research with animal/basic science studies. It is these animal/basic science studies that in all likelihood will provide the necessary advances toward understanding the true causes of ONJ and the most viable prevention/treatment strategies.
While a definitive cause/effect relationship between bisphosphonate treatment and ONJ has yet to be documented, compelling evidence exists in the literature [Zervas et al., 2006]. Working under the assumption of a direct connection, determining the underlying mechanism(s) connecting bisphosphonate treatment to ONJ becomes the top priority. The literature contains an exhaustive list of proposed mechanisms for ONJ, most of which have limited, or no, supportive data. The 3 most commonly proposed mechanisms include remodeling suppression, disrupted angiogenesis and infection; these are briefly reviewed below.
Remodeling Suppression
Bisphosphonate-induced remodeling suppression is by far the most often cited causative factor underlying the pathophysiology of ONJ despite the fact that there are no published data in humans showing the effects of bisphosphonates on jaw remodeling. Through their direct actions on osteoclasts, bisphosphonates significantly reduce bone remodeling [Rodan and Fleisch, 1996], the mechanism responsible for slowing bone loss in osteoporosis patients and lessening complications associated with skeletal metastases in cancer patients. Both human [Chavassieux et al., 1997; Eriksen et al., 2002; Recker et al., 2006] and animal studies [Balena et al., 1993; Smith et al., 2003; Allen et al., 2006] show that bisphosphonate doses used for osteoporosis treatment significantly suppress tissue-level bone remodeling (measured using fluorochrome-labeled bone histomorphometry). There are no data, in either humans or animals, describing the tissue-level remodeling effects of bisphosphonates when administered at doses used for cancer treatment. Such data are vital, because although measures of systemic remodeling suppression are similar between these 2 bisphosphonate dosing regimens [Chesnut et al., 1995; Berenson et al., 2001], different levels of remodeling suppression certainly could occur at the tissue level [Smith et al., 2003; Odvina et al., 2005]. This could help explain the greater incidence of ONJ in patients treated with bisphosphonates for cancer compared to those treated for osteoporosis.
It is important to note that the effects of bisphosphonates on tissue-level remodeling suppression are site specific with those sites that normally undergo higher rates of remodeling experiencing more marked suppression. In ovariectomized nonhuman primates, trabecular bone of the vertebrae remodels twice as fast as trabecular bone in the iliac crest and distal radius [Smith et al., 2003]. Treatment of these animals with ibandronate (10 μg/kg monthly, intravenously) suppresses trabecular remodeling by 75% in the vertebra, yet only by 20% in the iliac crest and distal radius [Smith et al., 2003]. Similar results were documented for cortical sites which differ in their normal remodeling rates; those with higher rates were more affected by bisphosphonates. These data have important implications for the jaw, which has been shown to have intracortical remodeling rates in humans that are 10–20 times higher than within the cortex of the iliac crest [Garetto et al., 1995; Han et al., 1997]. Remodeling rates in the jaw cortex can be even higher in the presence of infection or following dental intervention. With high remodeling rates that are unparalleled elsewhere in the skeleton, the jaw may be uniquely susceptible to remodeling suppression with bisphosphonates. Data from beagle dogs are consistent with this theory; etidronate, at doses 16 times higher than those used clinically, reduced alveolar bone intracortical remodeling from more than 40 to approximately 5% per year, while suppression at the rib (a cortical bone site with relatively rapid turnover) was reduced from 15 to 5% per year [Garetto and Tricker, 1998; Mashiba et al., 2001].
Angiogenesis
It is important to note that despite frequent use of the term avascular necrosis to describe ONJ in bisphosphonate-treated patients, there is no evidence that the necrotic regions have reduced vasculature or blood supply. Antiangiogenic effects of bisphosphonates have been documented by various groups, using both in vitro and in vivo experimental models. Wood et al. [2002] have shown that zoledronic acid significantly suppresses new vessel sprouting in culture and angiogenesis when tissue chambers are implanted subcutaneously in mice. These findings are supported by others showing reductions in tissue revascularization of rats and lower vessel densities in humans that had been treated with bisphosphonates [Fournier et al., 2002]. There are no data describing effects of bisphosphonates on angiogenesis in bone or bone marrow, the tissues of interest with respect to ONJ. Given the reductions in remodeling with bisphosphonates, suppression of angiogenesis within the bone matrix is expected as new remodeling units (and their associated vessels) would be initiated at a much slower rate. Reduced angiogenesis in this context would be considered secondary to the effects of remodeling suppression. Antiangiogenic effects of bisphosphonates could have a direct influence on soft tissue mucosa, vasculature of the bone marrow or in the wound-healing response that follows dental intervention, none of which have been studied. Some insight into wound healing may be gleaned from the fracture-healing literature, which shows that bisphosphonates allow normal callus formation [Peter et al., 1996; Li et al., 1999; Cao et al., 2002]. This suggests that early stages of wound healing (such as revascularization) are not adversely affected by bisphosphonates. However, these same studies show impairment of callus remodeling. Delayed remodeling, if also shown to occur in the oral cavity following tooth extraction, would be consistent with the ONJ literature.
Infection
The universal presence of Actinomyces in a case series of samples from ONJ patients strongly suggests a role of infection in ONJ [Hansen et al., 2006]. The oral cavity is home to hundreds of microorganisms, the risk of infection is increased following dental procedures and cancer patients are routinely treated with immunosuppressive agents. These factors may all come together to provide the perfect environment for chronic infection (osteomyelitis). How, or if, this contributes to ONJ is unclear; however, most evidence suggests the necrotic tissue becomes infected as opposed to the infected tissue becoming necrotic [Yarom et al., 2007]. Conflicting reports exist with respect to effects of bisphosphonates on immune cells. Bisphosphonates have been shown to inhibit T lymphocyte activation and proliferation as well as to suppress monocytes production of various pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α), which in turn affect antigen-presenting cells [Milhaud et al., 1983; Sansoni et al., 1995]. However, data also exist showing enhanced production of pro-inflammatory cytokines by lymphocytes (specifically γ/δ T cells) in response to bisphosphonates. This latter effect has been deemed responsible for the acute-phase reaction associated with intravenous bisphosphonate treatment [Hewitt et al., 2005; Coxon et al., 2006]. These limited data suggest that bisphosphonates do exert direct effects on cells of the immune system.
From the Bedside to the Bench: Working toward an Animal Model of ONJ
There are numerous limitations to studying ONJ in humans, emphasizing the need to transition toward animal/basic science studies. For nearly a decade, our laboratory has been studying the effects of bisphosphonates on tissue-level properties using a dog model. The dog offers numerous advantages as an animal model for studying skeletal physiology. The bone turnover rate of beagles is similar to humans, albeit with a slightly shorter remodeling period [Eriksen et al., 1994; Boyce et al., 1995]. Like humans, dogs also undergo intracortical remodeling (remodeling within cortical bone) which is essential in studies where cortical bone physiology is of interest. Rodents, under normal conditions, do not undergo appreciable amounts of intracortical bone remodeling. With the goal of understanding the effects of bisphosphonates on various tissue-level bone properties, our laboratory recently undertook a study in which female beagles were treated with clinically relevant doses of daily oral alendronate for 1 or 3 years [Allen et al., 2006; Allen and Burr, 2007]. Although the original aims of the study did not involve the craniofacial bones, mandibles from these animals were harvested and are now being analyzed to determine what effects bisphosphonates may have on the jaws of these animals [Allen and Burr, 2008].
Using dynamic histomorphometry, the level of intracortical bone remodeling in the mandible of untreated dogs in this experiment was found to be more than 10 times higher than within the tibial cortex of the same animals. Specifically, it was the alveolar portion of the mandible that had the highest rate of intracortical turnover, being more than 8 times higher than in the nonalveolar portions of the mandible. These results are similar to those previously shown in untreated dogs [Garetto and Tricker, 1998; Huja et al., 2006]. Treatment for 3 years with clinically relevant doses of alendronate significantly suppressed (–75%) the level of intracortical remodeling in the alveolar bone compared to vehicle-treated animals. These data support previous reports in dogs treated with etidronate [Garetto and Tricker, 1998], while extending the results to encompass clinically relevant dosing regimens. There was no significant effect of 3 years of alendronate on the remodeling rate of nonalveolar bone in the mandible. Interestingly, the most prominent suppression of remodeling in the alveolar bone is consistent with the anatomical location of the jaw bone most often associated with ONJ in humans.
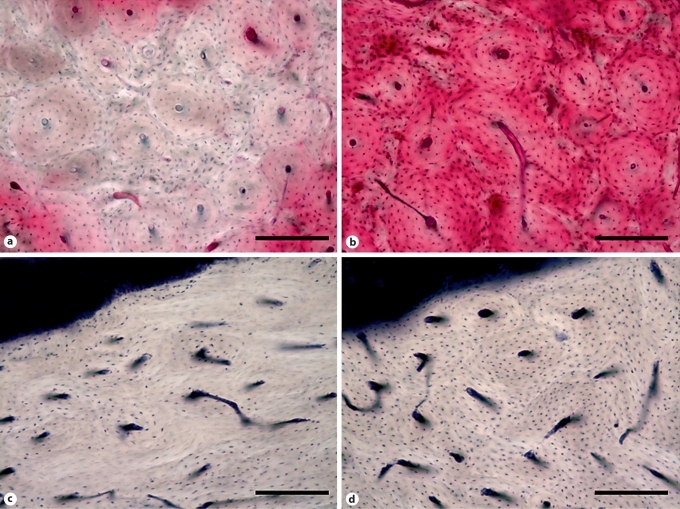
None of these dogs treated for 1 or 3 years with oral alendronate developed exposed lesions in the oral cavity. To determine if necrosis existed within the bone matrix of the mandible, segments were stained en bloc with basic fuchsin, a stain that passively diffuses into and fills all void spaces within the bone (Haversian canals, canaliculi, microdamage). This technique allows histological assessment of necrotic matrix regions as those regions which have lost patent canalicular/osteocyte networks are void of stain [Frost, 1960; Enlow, 1962, 1966]. We found significant amounts of necrotic bone matrix in approximately 25% of the bisphosphonate-treated animals that had been treated for 1 or 3 years (fig. 1) [Allen and Burr, 2008]. No such regions existed in any of the vehicle-treated animals. These regions averaged approximately 1 mm2 in size and were predominantly found in the alveolar portion of the mandible [Allen and Burr, 2008]. Again, the predominant localization of these necrotic regions to alveolar bone is consistent with the anatomical location of the jaw which undergoes the greatest amount of remodeling suppression (from these dog studies) and has the highest incidence of ONJ (from human studies).
Fig. 1.
Photomicrographs of devitalized bone matrix in the mandible of bisphosphonate-treated dogs. a Matrix necrosis, assessed using en bloc basic fuchsin staining, can be observed in the mandible of a dog treated daily for 1 year with oral alendronate, by the complete absence of fuchsin stain (red) in a localized region. b A region from the mandible of a vehicle-treated animal shows staining of viable tissue. c Focal loss of viable osteocytes, assessed using lactate dehydrogenase histochemistry which labels viable osteocytes blue, can be observed in the alveolar bone of an animal treated for 3 months with intravenous zoledronate. d A region from a vehicle-treated animal shows the majority of osteocytes are viable (stained blue). Scale bars = 200 μm.
Our laboratory is currently undertaking a follow-up study aimed at investigating whether intravenous zoledronate, at dosing regimens consistent with those used in cancer patients, differentially produces nonviable tissue in the mandible compared to oral alendronate. Preliminary observations of tissue from animals treated for 3 months show focal regions of nonviable osteocytes (assessed using lactate dehydrogenase histochemistry) in the alveolar bone of zoledronate-treated animals (fig. 1). Similar regions were not evident in the alveolar bone of animals treated with vehicle or alendronate in the small subset of animals examined to date (5 animals per group). Ongoing analyses will quantify osteocyte viability, matrix necrosis (using basic fuchsin staining) and bone remodeling following both 3 and 6 months of these treatments.
We hypothesize that the accumulation of nonviable osteocytes and matrix necrosis observed in these dogs is the result of suppressed intracortical remodeling and that they are part of the underlying pathophysiology of ONJ. Loss of osteocyte viability is a normal process [Jilka et al., 2007] and when a collection of cells becomes nonviable, the tissue is targeted for remodeling [Verborgt et al., 2002]. By suppressing turnover, regions of nonviable osteocytes are inevitably removed at a slower rate. This produces localized tissue necrosis, containing regions of nonviable osteocytes or, if allowed sufficient time, regions where the osteocytes and their canaliculi fill with mineral [Frost, 1960; Remaggi et al., 1996]. Presence of matrix necrosis in the alveolar bone of a tooth that undergoes dental extraction (or some other invasive dental procedure) is likely to contribute to a disrupted healing/remodeling response. The addition of infection to such a region would contribute, but would not be necessary, to further compromise healing and eventually result in significant devitalization of the region.
While these results are intriguing and suggest the dog may be a viable animal model to study ONJ, many questions remain unanswered. Specifically, it will be important to determine if focal matrix necrosis is part of the ONJ pathophysiology. Documenting a connection between matrix necrosis and exposed oral lesions, for instance with an additional intervention such as dental extraction, would establish whether this can be used as a model system to study ONJ. Once established, this or any other animal model would pave the way for a series of studies to help determine the specific role of remodeling suppression, angiogenesis and infection in the pathophysiology of ONJ and if/how the entity of ONJ can be treated and prevented.
Acknowledgements
The author would like to thank Dr. David Burr for his intellectual contributions to many of the concepts addressed herein. The animal studies and analyses discussed in this work were supported by funds from the National Institutes of Health, the National Osteoporosis Foundation, Eli Lilly Research Laboratories and Amgen. Merck kindly provided alendronate for the 1- and 3-year animal experiments.
Abbreviations used in this paper
- ONJ
osteonecrosis of the jaw
References
- Allen M.R., Iwata K., Phipps R., Burr D.B. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Allen M.R., Burr D.B. Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res. 2007;22:1759–1765. doi: 10.1359/jbmr.070720. [DOI] [PubMed] [Google Scholar]
- Allen M.R., Burr D.B. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. J Oral Maxillofac Surg. 2008;66:987–994. doi: 10.1016/j.joms.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balena R., Toolan B.C., Shea M., Markatos A., Myers E.R., Lee S.C., Opas E.E., Seedor J.G., Klein H., Frankenfield D. The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J Clin Invest. 1993;92:2577–2586. doi: 10.1172/JCI116872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson J.R., Vescio R.A., Rosen L.S., VonTeichert J.M., Woo M., Swift R., Savage A., Givant E., M. Hupkes, H. Harvey, A. Lipton. A phase I dose-ranging trial of monthly infusions of zoledronic acid for the treatment of osteolytic bone metastases. Clin Cancer Res. 2001;7:478–485. [PubMed] [Google Scholar]
- Boyce R.W., Paddock C.L., Gleason J.R., Sletsema W.K., Eriksen E.F. The effects of risedronate on canine cancellous bone remodeling: three-dimensional kinetic reconstruction of the remodeling site. J Bone Miner Res. 1995;10:211–221. doi: 10.1002/jbmr.5650100207. [DOI] [PubMed] [Google Scholar]
- Cao Y., Mori S., Mashiba T., Westmore M.S., Ma L., Sato M., Akiyama T., Shi L., Komatsubara S., Miyamoto K., Norimatsu H. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002;17:2237–2246. doi: 10.1359/jbmr.2002.17.12.2237. [DOI] [PubMed] [Google Scholar]
- Chavassieux P.M., Arlot M.E., Reda C., Wei L., Yates A.J., Meunier P.J. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997;100:1475–1480. doi: 10.1172/JCI119668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut C.H. 3rd, McClung M.R., Ensrud K.E., Bell N.H., Genant H.K., Harris S.T., F.R. Singer, J.L. Stock, R.A. Yood, P.D. Delmas, et al. Alendronate treatment of the postmenopausal osteoporotic woman: effect of multiple dosages on bone mass and bone remodeling. Am J Med. 1995;99:144–152. doi: 10.1016/s0002-9343(99)80134-x. [DOI] [PubMed] [Google Scholar]
- Coxon F.P., K. Thompson, M.J. Rogers. Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol. 2006;6:307–312. doi: 10.1016/j.coph.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Enlow D.H. Functions of the Haversian system. Am J Anat. 1962;110:269–305. doi: 10.1002/aja.1001100305. [DOI] [PubMed] [Google Scholar]
- Enlow D.H. Osteocyte necrosis in normal bone. J Dent Res. 1966;45:213. doi: 10.1177/00220345660450011901. [DOI] [PubMed] [Google Scholar]
- Eriksen E., Axelrod D., Melsen F. Bone Histomorphometry. New York: Raven Press; 1994. [Google Scholar]
- Eriksen E.F., Melsen F., Sod E., Barton I., Chines A. Effects of long-term risedronate on bone quality and bone turnover in women with postmenopausal osteoporosis. Bone. 2002;31:620–625. doi: 10.1016/s8756-3282(02)00869-4. [DOI] [PubMed] [Google Scholar]
- Fournier P., Boissier S., Filleur S., Guglielmi J., Cabon F., M. Colombel, P. Clezardin. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002;62:6538–6544. [PubMed] [Google Scholar]
- Frost H.M. Micropetrosis. J Bone Joint Surg Am. 1960;42A:144–150. [PubMed] [Google Scholar]
- Garetto L.P., Chen J., Parr J.A., Roberts W.E. Remodeling dynamics of bone supporting rigidly fixed titanium implants: a histomorphometric comparison in four species including humans. Implant Dent. 1995;4:235–243. doi: 10.1097/00008505-199500440-00002. [DOI] [PubMed] [Google Scholar]
- Garetto L.P., Tricker N.D. Remodeling of bone surrounding the implant interface. In: Garetto, L.P., Turner C.H., Duncan R.L., Burr D.B., editors. Bridging the Gap between Dental and Orthopaedic Implants: Proceedings of the 3rd Annual Indiana Conference, Indianapolis, Indiana. Indianapolis: Indiana University School of Dentistry and Indiana University School of Medicine; 1998. [Google Scholar]
- Han Z.H., Palnitkar S., Rao D.S., Nelson D., Parfitt A.M. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res. 1997;12:498–508. doi: 10.1359/jbmr.1997.12.4.498. [DOI] [PubMed] [Google Scholar]
- Hansen T., Kunkel M., Weber A., James Kirkpatrick C. Osteonecrosis of the jaws in patients treated with bisphosphonates – histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med. 2006;35:155–160. doi: 10.1111/j.1600-0714.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- Hewitt R.E., Lissina A., Green A.E., Slay E.S., Price D.A., Sewell A.K. The bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral blood gd T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol. 2005;139:101–111. doi: 10.1111/j.1365-2249.2005.02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huja S.S., Fernandez S.A., Hill K.J., Li Y. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1243–1249. doi: 10.1002/ar.a.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka R.L., Weinstein R.S., Parfitt A.M., Manolagas S.C. Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J Bone Miner Res. 2007;22:1492–1501. doi: 10.1359/jbmr.070518. [DOI] [PubMed] [Google Scholar]
- Li J., Mori S., Kaji Y., Mashiba T., Kawanishi J., Norimatsu H. Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. J Bone Miner Res. 1999;14:969–979. doi: 10.1359/jbmr.1999.14.6.969. [DOI] [PubMed] [Google Scholar]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- Mashiba T., Turner C.H., Hirano T., Forwood M.R., Jacob D.S., Johnston C.C., Burr D.B. Effects of high-dose etidronate treatment on microdamage accumulation and biomechanical properties in beagle bone before occurrence of spontaneous fractures. Bone. 2001;29:271–278. doi: 10.1016/s8756-3282(01)00575-0. [DOI] [PubMed] [Google Scholar]
- Mehrotra B., Fantasia J., Nissel-Horowitz S., Vinarsky S., Sheth M., Ruggiero S. Osteonecrosis of the maxilla: an unusual complication of prolonged bisphosphonate therapy. a case report. Proc Am Soc Clin Oncol. 2003;22:3194. [Google Scholar]
- Milhaud G., Labat M.L., Moricard Y. (Dichloromethylene)diphosphonate-induced impairment of T-lymphocyte function. Proc Natl Acad Sci USA. 1983;80:4469–4473. doi: 10.1073/pnas.80.14.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odvina C.V., Zerwekh J.E., Rao D.S., Maalouf N., Gottschalk F.A., Pak C.Y. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- Peter C.P., Cook W.O., Nunamaker D.M., Provost M.T., Seedor J.G., Rodan G.A. Effect of alendronate on fracture healing and bone remodeling in dogs. J Orthop Res. 1996;14:74–79. doi: 10.1002/jor.1100140113. [DOI] [PubMed] [Google Scholar]
- Recker R.R., Boonen S., Garcia P., Supronik J., Peichl P., Black D., Krasnow J., Chiodo J., J.E.E. Gasser. The effect of annual treatment with zoledronic acid 5 mg on bone remodeling: bone histomorphometry results from the HORIZON-PFT. J Bone Miner Res. 2006;21:S290. [Google Scholar]
- Remaggi F., Ferretti M., Cane V., Zaffe D. Histomorphological and chemico-physical analyses of the mineral matrix of micropetrotic human bone. Ann Anat. 1996;178:223–227. doi: 10.1016/S0940-9602(96)80052-5. [DOI] [PubMed] [Google Scholar]
- Rodan G.A., Fleisch H.A. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansoni P., Passeri G., Fagnoni F., Mohagheghpour N., Snelli G., Brianti V., Engleman E.G. Inhibition of antigen-presenting cell function by alendronate in vitro. J Bone Miner Res. 1995;10:1719–1725. doi: 10.1002/jbmr.5650101115. [DOI] [PubMed] [Google Scholar]
- Smith S.Y., Recker R.R., Hannan Muller M., Bauss F. Intermittent intravenous administration of the bisphosphonate ibandronate prevents bone loss and maintains bone strength and quality in ovariectomized cynomolgus monkeys. Bone. 2003;32:45–55. doi: 10.1016/s8756-3282(02)00923-7. [DOI] [PubMed] [Google Scholar]
- Verborgt O., Tatton N.A., Majeska R.J., Schaffler M.B. Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? J Bone Miner Res. 2002;17:907–914. doi: 10.1359/jbmr.2002.17.5.907. [DOI] [PubMed] [Google Scholar]
- Wood J., Bonjean K., Ruetz S., Bellahcene A., Devy L., Foidart J.M., V. Castronovo, J.R. Green. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055–1061. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- Yarom N., Yahalom R., Shoshani Y., Hamed W., Regev E., Elad S. Osteonecrosis of the jaw induced by orally administered bisphosphonates: incidence, clinical features, predisposing factors and treatment outcome. Osteoporos Int. 2007;18:1363–1370. doi: 10.1007/s00198-007-0384-2. [DOI] [PubMed] [Google Scholar]
- Zervas K., Verrou E., Teleioudis Z., Vahtsevanos K., Banti A., Mihou D., Krikelis D., Terpos E. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single-centre experience in 303 patients. Br J Haematol. 2006;134:620–623. doi: 10.1111/j.1365-2141.2006.06230.x. [DOI] [PubMed] [Google Scholar]