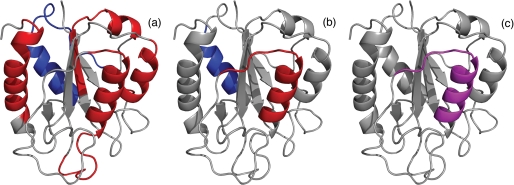
FIGURE 5.
Several folding properties of flavodoxin are highlighted in schematic diagrams of the native protein. a, the four regions of unfolded flavodoxin in 3.4 m GdnHCl that have restricted flexibility on the (sub)nanosecond time scale (44) are colored red; assembly of these structured elements leads to formation of the ordered core of the flavodoxin molten globule (43). The region of the molten globule that remains random coil down to a GdnHCl concentration of 1.58 m (i.e. residues Lys13–Val36) (43) is colored blue. b, residues Leu110–Val125 of the molten globule of flavodoxin have the highest protection factors against H/D exchange and are colored red; the region of the molten globule that probably exchanges its backbone amides via an EX1 mechanism, as determined in this study, is colored blue. In unfolded flavodoxin in 3.4 m GdnHCl, residues Glu104–Lys118 transiently form an α-helix (44). c, partially unfolded form PUF4 of apoflavodoxin as detected by native state H/D exchange (25). In PUF4, only the amides of the residues that are colored purple are protected against H/D exchange, and all other parts of the protein are unfolded.