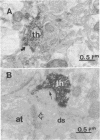
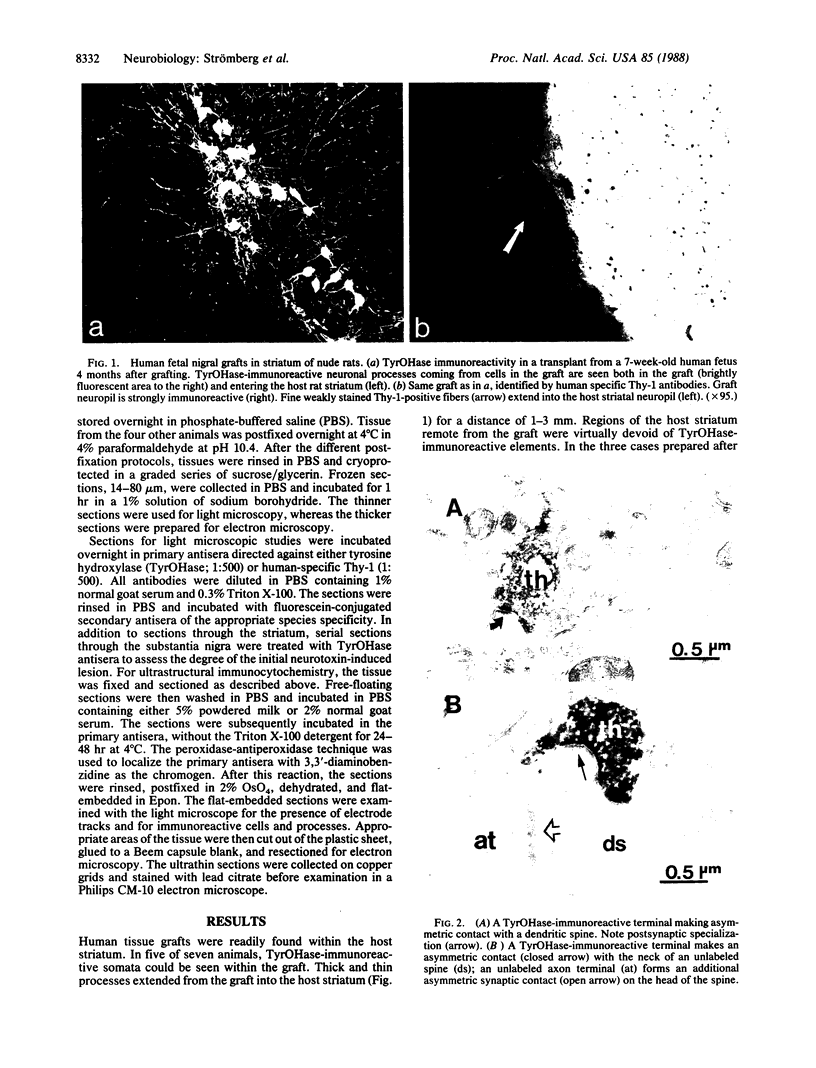
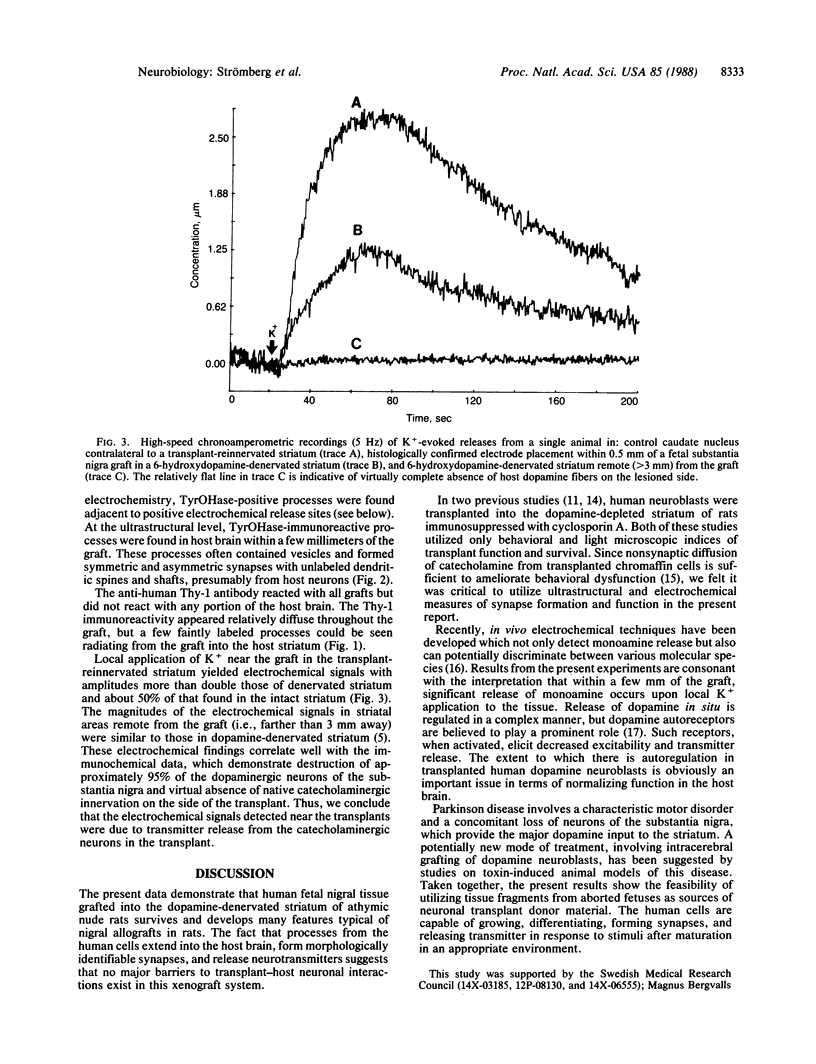
Abstract
Intracerebral allografts of fetal neurons have been studied in both rodents and nonhuman primates. Such research has been directed towards problems in developmental neurobiology and in animal models of neurological diseases. Whether intracerebrally transplanted human fetal neurons are capable of forming synapses and releasing neurotransmitters are key questions in any application of this approach to human brain development and dysfunction. We studied these questions by examining the immunocytochemical and in vivo electrochemical properties of xenografts of human mesencephalic dopaminergic neurons placed into athymic "nude" rats. The transplanted neurons survive, continue to express human-specific Thy-1 immunoreactivity, and extend neuronal processes into the host brain where morphologically identifiable synapses form. Potassium-evoked release of monoamines occurs in the vicinity of the graft but is absent in more remote areas of the host neuropil. These results indicate that human fetal tissue fragments can provide a source of viable neuroblasts for transplantation. Further, synapses form between pre- and postsynaptic elements expressing different species-specific cell surface markers; thus, these markers do not play a determining role in synaptogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannon M. J., Roth R. H. Pharmacology of mesocortical dopamine neurons. Pharmacol Rev. 1983 Mar;35(1):53–68. [PubMed] [Google Scholar]
- Björklund A., Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979 Nov 30;177(3):555–560. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- Brundin P., Nilsson O. G., Strecker R. E., Lindvall O., Astedt B., Björklund A. Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson's disease. Exp Brain Res. 1986;65(1):235–240. doi: 10.1007/BF00243848. [DOI] [PubMed] [Google Scholar]
- Freed W. J., Ko G. N., Niehoff D. L., Kuhar M. J., Hoffer B. J., Olson L., Cannon-Spoor H. E., Morihisa J. M., Wyatt R. J. Normalization of spiroperidol binding in the denervated rat striatum by homologous grafts of substantia nigra. Science. 1983 Nov 25;222(4626):937–939. doi: 10.1126/science.6635666. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Bolam J. P., Björklund A., Stenevi U., Dunnett S. B., Powell J. F., Smith A. D. Efferent synaptic connections of grafted dopaminergic neurons reinnervating the host neostriatum: a tyrosine hydroxylase immunocytochemical study. J Neurosci. 1985 Mar;5(3):603–616. doi: 10.1523/JNEUROSCI.05-03-00603.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt G. A., Gratton A., Rose G. M. In vivo electrochemical studies of the effects of cocaine on dopamine nerve terminals in the rat neostriatum. Physiol Bohemoslov. 1988;37(3):249–257. [PubMed] [Google Scholar]
- Gerhardt G. A., Oke A. F., Nagy G., Moghaddam B., Adams R. N. Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res. 1984 Jan 9;290(2):390–395. doi: 10.1016/0006-8993(84)90963-6. [DOI] [PubMed] [Google Scholar]
- Gerhardt G. A., Rose G. M., Hoffer B. J. Release of monoamines from striatum of rat and mouse evoked by local application of potassium: evaluation of a new in vivo electrochemical technique. J Neurochem. 1986 Mar;46(3):842–850. doi: 10.1111/j.1471-4159.1986.tb13048.x. [DOI] [PubMed] [Google Scholar]
- Jaeger C. B. Cytoarchitectonics of substantia nigra grafts: a light and electron microscopic study of immunocytochemically identified dopaminergic neurons and fibrous astrocytes. J Comp Neurol. 1985 Jan 1;231(1):121–135. doi: 10.1002/cne.902310110. [DOI] [PubMed] [Google Scholar]
- Mahalik T. J., Finger T. E., Stromberg I., Olson L. Substantia nigra transplants into denervated striatum of the rat: ultrastructure of graft and host interconnections. J Comp Neurol. 1985 Oct 1;240(1):60–70. doi: 10.1002/cne.902400105. [DOI] [PubMed] [Google Scholar]
- Perlow M. J., Freed W. J., Hoffer B. J., Seiger A., Olson L., Wyatt R. J. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979 May 11;204(4393):643–647. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- Redmond D. E., Sladek J. R., Jr, Roth R. H., Collier T. J., Elsworth J. D., Deutch A. Y., Haber S. Fetal neuronal grafts in monkeys given methylphenyltetrahydropyridine. Lancet. 1986 May 17;1(8490):1125–1127. doi: 10.1016/s0140-6736(86)91839-8. [DOI] [PubMed] [Google Scholar]
- Rose G., Gerhardt G., Strömberg I., Olson L., Hoffer B. Monoamine release from dopamine-depleted rat caudate nucleus reinnervated by substantia nigra transplants: an in vivo electrochemical study. Brain Res. 1985 Aug 19;341(1):92–100. doi: 10.1016/0006-8993(85)91476-3. [DOI] [PubMed] [Google Scholar]
- Strömberg I., Bygdeman M., Goldstein M., Seiger A., Olson L. Human fetal substantia nigra grafted to the dopamine-denervated striatum of immunosuppressed rats: evidence for functional reinnervation. Neurosci Lett. 1986 Nov 21;71(3):271–276. doi: 10.1016/0304-3940(86)90632-4. [DOI] [PubMed] [Google Scholar]
- Strömberg I., Herrera-Marschitz M., Ungerstedt U., Ebendal T., Olson L. Chronic implants of chromaffin tissue into the dopamine-denervated striatum. Effects of NGF on graft survival, fiber growth and rotational behavior. Exp Brain Res. 1985;60(2):335–349. doi: 10.1007/BF00235929. [DOI] [PubMed] [Google Scholar]
- Strömberg I., Johnson S., Hoffer B., Olson L. Reinnervation of dopamine-denervated striatum by substantia nigra transplants: immunohistochemical and electrophysiological correlates. Neuroscience. 1985 Apr;14(4):981–990. doi: 10.1016/0306-4522(85)90270-2. [DOI] [PubMed] [Google Scholar]
- Zetterström T., Brundin P., Gage F. H., Sharp T., Isacson O., Dunnett S. B., Ungerstedt U., Björklund A. In vivo measurement of spontaneous release and metabolism of dopamine from intrastriatal nigral grafts using intracerebral dialysis. Brain Res. 1986 Jan 8;362(2):344–349. doi: 10.1016/0006-8993(86)90460-9. [DOI] [PubMed] [Google Scholar]