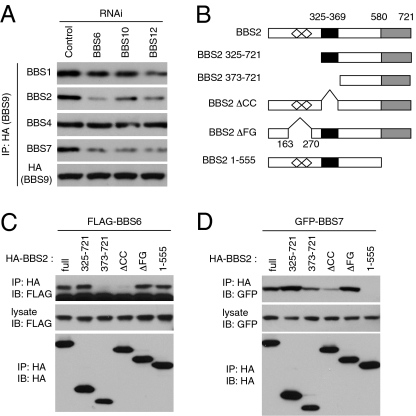
Fig. 3.
Chaperonin-like BBS proteins are required for BBSome assembly. (A) BBSome assembly in BBS6-, BBS10-, and BBS12-depleted cells. HA-BBS9 was transfected into control, BBS6-, BBS10-, and BBS12-depleted HEK293T cells, and BBSome assembly was assessed by measuring BBSome subunits associated with BBS9. (B) BBS2 deletion mutant constructs used to map BBS6- and BBS7-interacting domains. Open diamonds represent FG-GAP motifs (FG); black box indicates the coiled-coil (CC) domain; and gray box indicates the C-terminal α-helix–rich domain. Numbers represent amino acid residues. (C) Coiled-coil domain in BBS2 interacts with BBS6. HA-tagged BBS2 deletion mutants were cotransfected with FLAG-BBS6 into HEK293T cells, and lysates were immunoprecipitated with anti-HA antibodies. (D) C-terminal domain in BBS2 interacts with BBS7, but BBS6-interacting domain is required for efficient binding with BBS7. GFP-BBS7 was cotransfected with BBS2 deletion mutants.