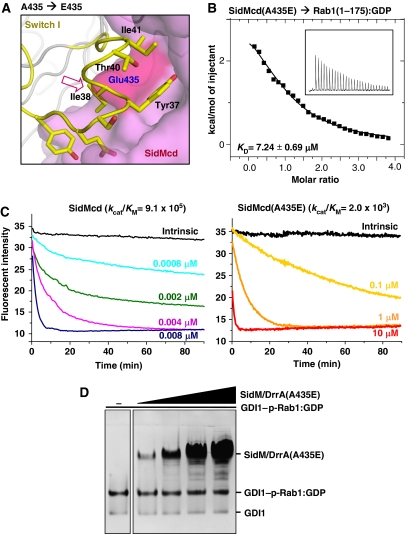
Figure 3.
Both GEF and GDF activities of SidM/DrrA are affected by a single mutation on its Rab1-interacting interface. (A) Structure-based design of a SidM/DrrA mutant. SidMcd is in surface representation and the Rab1 residues are represented by sticks. The red arrow indicates that glutamic acid at the position of A435 of SidM/DrrA is sterically incompatible for interacting with Rab1. (B) The A435E mutation reduces the binding affinity between SidMcd and Rab1(1-175):GDP. The ITC run and the deduced Kd are shown. (C) The A435E mutation reduces the GEF activity of SidMcd. In the presence of 0.2 mM GTP, Rab1:mant-GDP was incubated with wild-type SidMcd or SidMcd(A435E) at the indicated concentrations. The decreased fluorescence as a result of the mant-GDP-to-GTP exchange was continuously monitored and used to deduce the kcat/KM values (M−1 s−1), as reported previously (Murata et al, 2006). (D) On the native gel, the SidM/DrrA(A435E) mutant exhibits barely detectable GDF activity.