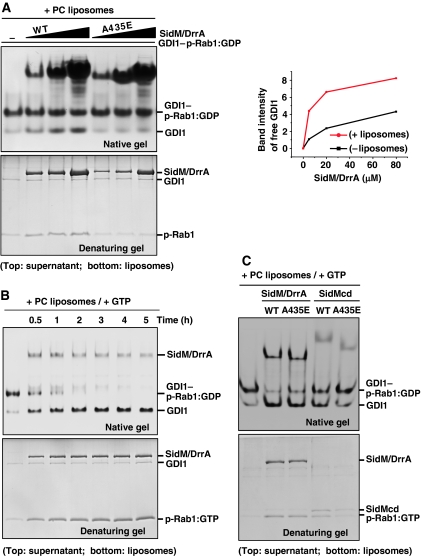
Figure 4.
Effects of PC liposomes and GTP on GDI displacement by SidM/DrrA. (A) Effect of PC liposomes. GDI1–p-Rab1:GDP (5 μM) was incubated for 5 h with wild-type or A435E mutant SidM/DrrA at 5, 20 or 80 μM in the presence of 2 mM PC liposomes. After centrifugation, the supernatant and liposome fractions were visualized on a native or denaturing gel. The A435E mutant exhibits barely detectable GDI1 displacement. The right panel shows quantification of the band intensities of released GDI1 (lanes 1–4 in Figures 2B and 4A). Wild-type SidM/DrrA exhibits higher GDF activity compared with its activity in the absence of the liposomes. (B) Effect of the presence of both PC liposomes and GTP. GDI1–p-Rab1:GDP (5 μM) was incubated with wild-type SidM/DrrA (3.5 μM) up to 5 h in the presence of 2 mM PC liposomes and 1 mM GTP. The native gel shows complete displacement of GDI1 from p-Rab1 in 4 h with concomitant enrichment of p-Rab1 in the liposome fraction (denaturing gel). Lane 1 is for control showing GDI1–p-Rab1:GDP complex incubated for 5 h without added SidM/DrrA. (C) GDF activity assay involving the SidM/DrrA(A435E) and SidMcd(A435E) mutants. GDI1–p-Rab1:GDP (5 μM) was incubated with 5 μM of wild-type SidM/DrrA, SidM/DrrA(A435E), wild-type SidMcd or SidMcd(A435E) for 30 min in the presence of 2 mM PC liposomes and 1 mM GTP. Displacement of GDI1 is observable for the two mutants. Regardless of the mutation, full-length protein is more active than the central domain.