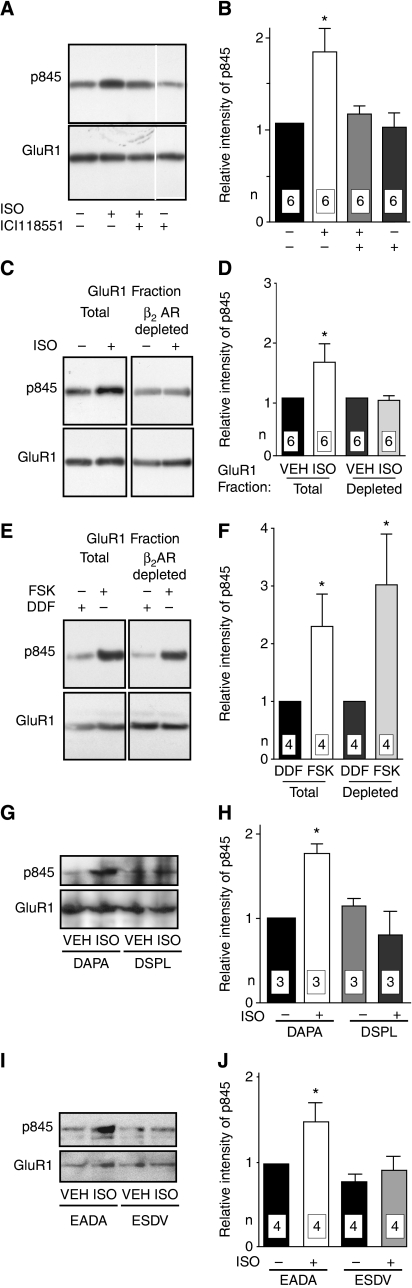
Figure 3.
Localized regulation of GluR1 S845 phosphorylation by the β2AR. Primary hippocampal cultures (18 DIV) were treated with vehicle (VEH), 3 μM ISO, 10 μM forskolin (FSK), or 10 μM 1,9-dideoxyforskolin (DDF; inactive forskolin homologue) for 15 min. Cultures were extracted with Triton X-100 and cleared by ultracentrifugation. GluR1 was immunoprecipitated directly or after pre-immunoprecipitation of β2AR complexes (H-20) before immunoblotting with antibodies against phosphorylated S845 (top) and total GluR1 (bottom). Immunosignals were quantified by densitometry. Phospho-S845 (pS845) signals were corrected with respect to total GluR1 signals and normalized to control. (A, B) ISO significantly increased S845 phosphorylation. Pre-treatment for 15 min with 1 μM ICI118551-blocked ISO-induced phosphorylation. All lanes are from same blot and exposure, but non-relevant lanes have been removed between lane 3 and 4. (C–F) After treatments, culture extracts were split into two equal portions. In contrast to the total GluR1 population (left pairs in C, D), GluR1 remaining after depletion of GluR1–β2AR complexes by pre-immunoprecipitation with H-20 showed no ISO-induced increase in S845 phosphorylation (right pairs in C, D), although forskolin stimulated S845 phosphorylation in both the total and the GluR1–β2AR-depleted GluR1 populations (E, F). (G–J) Pre-treatment for 2 h with membrane-permeant DSPL and ESDV peptides, but not their inactive analogues DAPA and EADA (1 μM each) prevented induction of S845 phosphorylation by ISO. *P<0.05 compared with control treatments; error bars: s.e.m.; n: number of independent experiments.