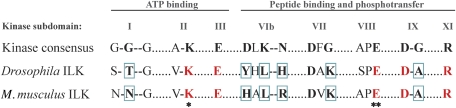
Figure 1.
The kinase homology domain of ILK lacks several critical residues present in eukaryotic serine/threonine kinases. Alignment of highly conserved amino acids within the 12 subdomains of eukaryotic serine/threonine kinases (Hanks et al, 1988) with Drosophila and Mus musculus ILK. Conserved amino acids are marked in bold, and their counterparts in the ILK sequences are shown in red. Conserved amino acids that are not present in the ILK sequences are highlighted with blue boxes. Most importantly, ILK lacks the catalytic base in subdomain VIb. Substitution of the invariant lysine in subdomain II (ATP binding) to either an alanine or methionine (K220A/M in M. musculus ILK; marked with *) or of the invariant glutamate in subdomain VIII (substrate recognition) to a lysine (E359K in M. musculus ILK; marked with **) have been shown to render ILK catalytically inactive.