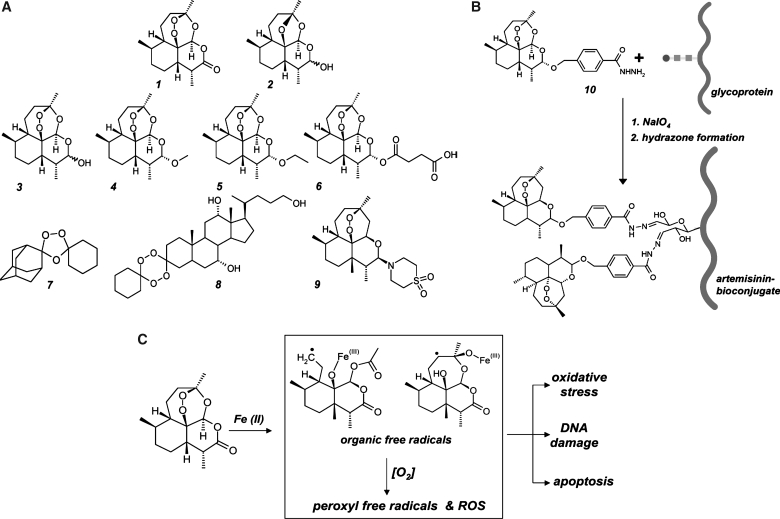
FIG. 3.
Artemisinins: A novel class of endoperoxide-based anticancer redox chemotherapeutics. (A) The sesquiterpene endoperoxide artemisinin (1) and 1,2,4-trioxane-based, semisynthetic artemisinin-derivatives including dihydroartemisinin (3), artemether (4), arteether (5), artesunate (6), and artemisone (9) are established prooxidant antimalarial drugs and investigational chemotherapeutics for oncological indications. Deoxyartemisinin (2) is a redox-inactive artemisinin derivative. OZ277 (7) and the steroidal tetraoxane 5β-cholan-7α,12α,24-triol-3-spiro-6′-(1′,2′,4′,5′-tetraoxacyclohexane)-3′-spiro-cyclo-hexane (8), are fully synthetic drug-like 1,2,4-trioxolane-derivatives. (B) Artemisinin–glycoprotein bioconjugates for targeted prooxidant intervention. Artemisinin bioconjugation of transferrin and other glycoproteins (curved line: protein; squares and circle: oligosaccharide chain) occurs via hydrazone formation using artelinic acid hydrazide (10) after periodate-induced oxidation of vicinal sugar diol-groups. (C) The free radical mechanism of artemisinin drug action. The pharmacophoric moiety of artemisinins is an endoperoxide bridge that can be fragmented by intracellular iron [Fe(II)] species, leading to the formation of carbon-centered electrophilic radical species including a sterically unhindered primary radical (middle panel, upper left) and a sterically more congested secondary radical (middle panel, upper right). These organic free racicals together with ROS formed through Fenton chemistry are the suggested key mediators of artemisinin-induced cancer cell inactivation.