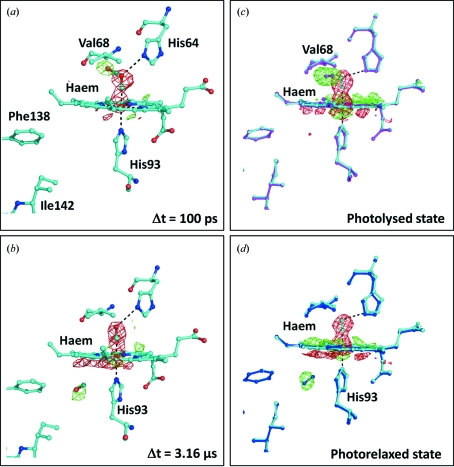
Figure 1.
Time-resolved Laue diffraction and intermediate trapping studies of the photodissociation of carbon monoxide bound to the haem group of myoglobin. (a) F obs(light) − F obs(dark) difference Fourier electron density map of myoglobin (L29F) calculated for the time point 100 ps following photoactivation by a short laser pulse (Schotte et al., 2003 ▶; Aranda et al., 2006 ▶). Negative difference electron density (red) is observed 100 ps after photoactivation at the resting-state position of the carbon monoxide molecule (above the haem) and positive difference electron density (green) is observed nearby below Val68. (b) A similar map calculated for the time point 3.16 µs after photoactivation. At this time another binding pocket is visible as positive difference electron density (green) below the haem group near His93. In calculating these maps the crystallographic observations were taken from Protein Data Bank entries 2g0s (resting state), 2g0v (100 ps) and 2g14 (3.16 µs) (Aranda et al., 2006 ▶). (c) Difference Fourier maps calculated from low-temperature trapping studies on wild-type myoglobin:carbon monoxide complexes (Chu et al., 2000 ▶). The resting-state model is shown in cyan (Protein Data Bank entry 1dwr) and the photolysed state is shown in magenta (1dws). (d) Difference Fourier map calculated for the photorelaxed state, shown in blue (1dwt), from the same low-temperature study. All maps are contoured at 4.5σ. Agreement is apparent between the positions of the carbon monoxide molecule observed in the intermediate trapping and the Laue diffraction studies.