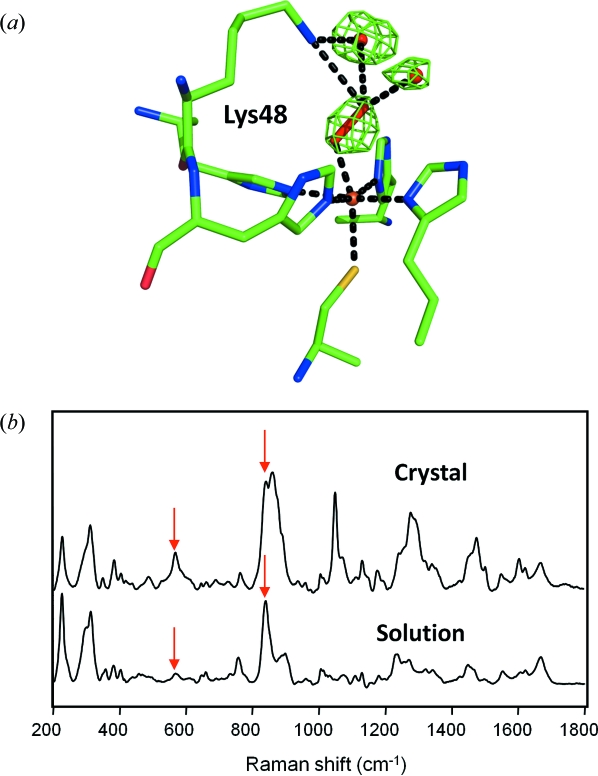
Figure 2.
Intermediate trapping studies of the non-haem iron protein superoxide reductase from D. barsii (Katona et al., 2007 ▶). (a) Structure of the iron peroxide intermediate bound in an end-on configuration in the active site of superoxide reductase. The F obs − F calc omit (green) maps are contoured at 4.5σ. The peroxo moiety is hydrogen-bonded to Lys48 and two water molecules of the active site, which assist the protonation en route to the product formation. The structural model and electron density derive from entry 2ji3 of the Protein Data Bank. (b) Off-resonance Raman spectra collected from single crystals and solutions of hydrogen-peroxide-treated superoxide reductase. Single crystals of superoxide reductase (top spectrum) were treated in crystallization buffer with the addition of 10 mM H2O2 for 3 min and subsequently flash frozen in a cryoprotected buffer. SOR solutions (bottom spectrum) were treated similarly (for details see Katona et al., 2007 ▶).