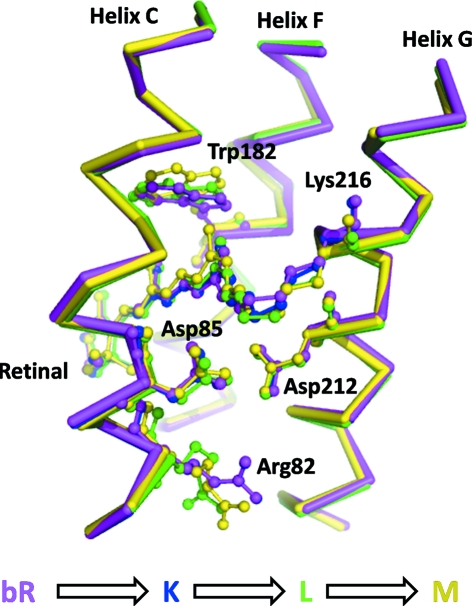
Figure 3.
Structural results from intermediate trapping studies of bacteriorhodopsin. Four structures of resting (Belrhali et al., 1999 ▶) (purple, Protein Data Bank entry 1qhj), early (Edman et al., 1999 ▶) (blue, 1qkp), intermediate (Royant et al., 2000 ▶) (green, 1eop) and late (Luecke et al., 1999 ▶) (yellow, 1c8s) conformations are shown. These intermediate conformations were trapped by illuminating crystals at 110 K, 170 K and during thawing, respectively. A clear evolution of the retinal can be observed for these structures, which moves towards the cytoplasm as the temperature is raised. Moreover, significant displacements of Trp-182, Asp-85 and Arg-82 are also observed, as are rearrangements of water molecules recorded in the corresponding Protein Data Bank entries (not shown for reasons of clarity).