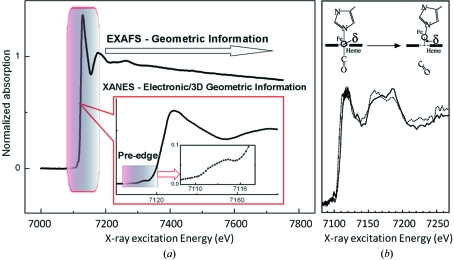
Figure 6.
X-ray absorption spectra from iron-containing proteins. (a) Static X-ray absorption spectra from the iron K edge of PerR protein (Jacquamet et al., 2009 ▶). Extended X-ray absorption fine structure (EXAFS) records the modulation of an X-ray absorption spectrum in the energy region from 50 eV to approximately 1000 eV above the absorption edge, providing structural information on the neighbours of the absorbing atom and very accurate first-shell iron–ligand distances. X-ray absorption near-edge structure (XANES) focuses upon the smaller energy region up to approximately 50 eV above the edge and provides structural and electronic information for the absorbing atom. The pre-edge region of XANES is sensitive to the oxidation, spin state and geometric environment of the absorbing atom. (b) Time-resolved X-ray absorption spectra of myoglobin in complex with carbon monoxide and its interpretation in terms of structure. Iron K-edge XANES spectra (dashed line) were recorded 100 µs following photoexcitation, and spectra from the resting conformation are shown for comparison (black line). Spectral changes were interpreted as resulting from the movement of carbon monoxide away from the haem group (inset). Reprinted from Journal of Electron Spectroscopy and Related Phenomena (Wang et al., 2005 ▶), copyright (2005), with permission from Elsevier.