Figure 1. NFAT3 is a novel RSK2-interacting protein.
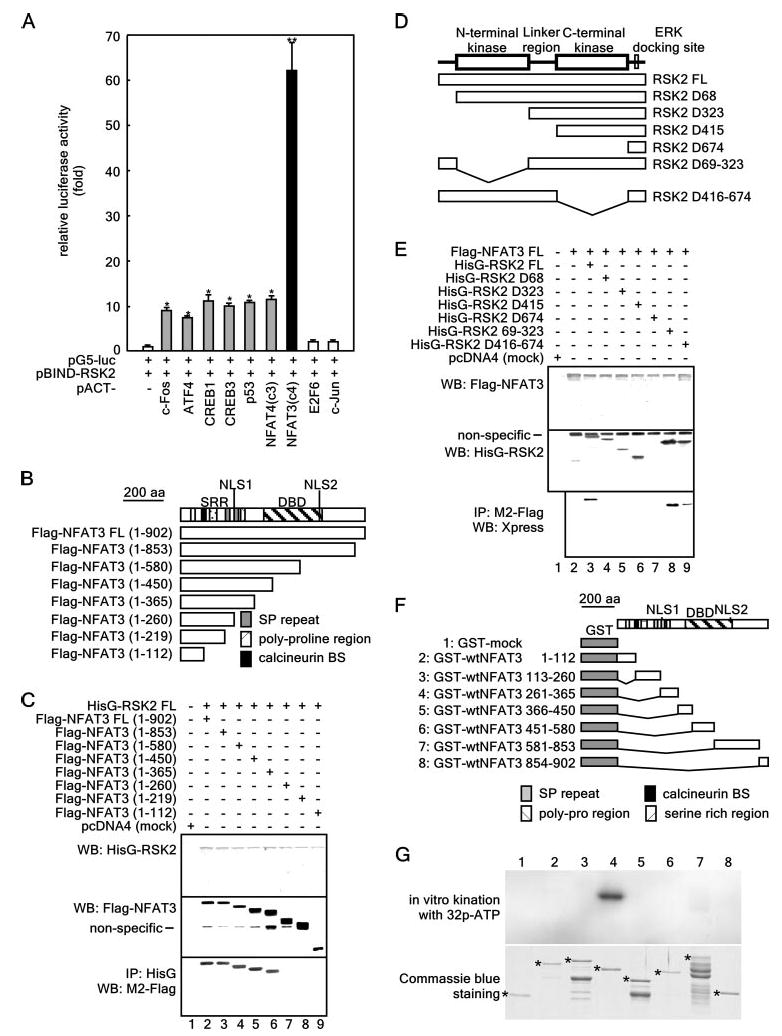
A, assessment of the in vivo protein-protein interactions of pBIND-RSK2 and pACT-TF plasmids by the mammalian two-hybrid assay. Activity is expressed as relative luminescence units normalized to a negative control (value for cells transfected with only pG5-luciferase (luc)/pBIND-RSK2 = 1.0). Data are expressed as the means ± S.D. of values obtained from triplicate experiments. Significant differences were evaluated using Student's t test (*, p < 0.01;**, p < 0.0001). ATF4, activating transcription factor-4. B, structure and schematic diagrams of NFAT3 deletion mutant constructs. SRR, serine-rich region; DBD, DNA-binding domain; SP repeat, Ser/Pro repeat; BS, binding site. C, identification of the NFAT3 domain that binds RSK2. To identify the domain of NFAT3 to which RSK2 binds, eight pcDNA3-FLAG-NFAT3 deletion constructs were individually cotransfected with pcDNA4-HisG-RSK2FL into 293 cells. After culturing for 48 h, cells were disrupted with Nonidet P-40 cell lysis buffer and immunoprecipitated (IP) with anti-HisG monoclonal antibody. NFAT3 was detected by Western blotting (WB) using HRP-conjugated anti-FLAG antibody M2. D, structure and schematic diagrams of RSK2 deletion mutant constructs. E, identification of the domain of RSK2 that binds NFAT3. To identify the domain of RSK2 to which NFAT3 binds, seven pcDNA4-RSK2 deletion constructs were individually cotransfected with pcDNA3-FLAG-NFAT3FL into 293 cells. After culturing for 48 h, cells were disrupted with Nonidet P-40 cell lysis buffer and immunoprecipitated with anti-FLAG monoclonal antibody M2. RSK2 was detected by Western blotting using HRP-conjugated anti-Xpress antibody. F, structure and schematic diagrams of GST-NFAT3 fusion constructs. G, target domain mapping of NFAT3. To identify the target domain of NFAT3 for RSK2, each GST-NFAT3 fusion protein was partially purified, directly subjected to an in vitro phosphorylation assay with active RSK2, and then visualized by autoradiography. Each asterisk indicates the respective GST fusion protein.
