Figure 6.
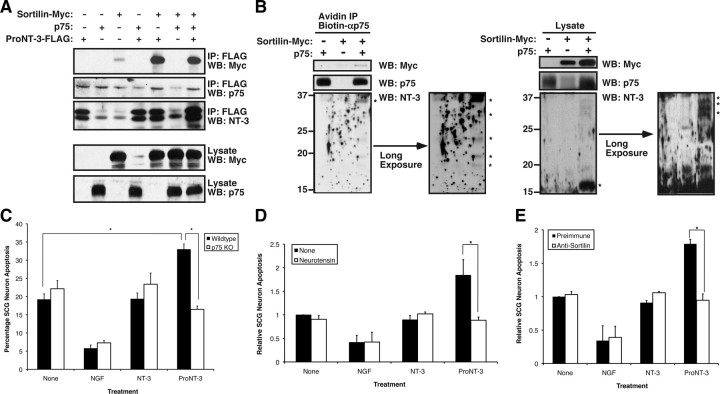
Dual-receptor requirement for ProNT-3 action. A, Coimmunoprecipitation of proNT-3 and sortilin. HEK 293T cells were transfected with Myc-tagged sortilin, p75NTR, FLAG-tagged proNT-3 alone or in combination as indicated. Forty-eight hours later, detergent lysates were immunoprecipitation with anti-FLAG beads (Sigma-Aldrich), followed by Western blotting analysis with anti-NT-3, anti-p75NTR, or anti-Myc antisera as indicated. Note that sortilin but not p75NTR readily coimmunoprecipitated with proNT-3 and that stable complex between sortilin and proNT-3 was not enhanced by p75NTR. B, Sortilin expression is required for proNT-3 binding to p75NTR. HEK 293T cells were transiently transfected with either p75NTR, Myc-tagged sortilin, or both receptors as indicated. Forty-eight hours later, exogenous recombinant proNT-3 (for proNT-3 production in 293T cells, see Fig. 3A) was added to these cells for 1 h. Left panel, Anti-p75NTR immunoprecipitation was performed as described (see Materials and Methods), followed by Western blotting for proNT-3 (anti-NT3), sortilin (anti-Myc), and p75NTR. Right panel, The corresponding total cellular lysates were similarly Western blotted with the indicated antisera. Note that proNT-3 internalization, which occurred only in cells that coexpress both p75NTR and sortilin, also resulted in significant degradation of the molecule. The numbers on the left indicate positions of the molecular weight markers. The asterisks (*) on the left indicate full-length proNT-3 (∼37 kDa) and various partially cleaved NT-3 species (∼32–16 kDa). Note also that, in these experiments, no mature NT-3 of the expected molecular weight (i.e., 13 kDa) was detected, suggesting that endocytosed proNT-3 is not processed by the same furin-based mechanism for mature NT-3 production. C, p75NTR is required for proNT-3-induced neuronal apoptosis. Wild-type or p75NTR-null SCG neurons were cultured as described previously (Nykjaer et al., 2004; Teng et al., 2005). Replica cultures (DIV 7), washed free of NGF, were treated or not with 10 ng/ml NGF or equal molar of NT-3 or proNT-3 (i.e., 2 ng/ml NT-3 or 4 ng/ml proNT-3) as indicated. The percentage of apoptotic neurons were assessed 36 h later. The data were not further normalized because neurons for these experiments were from two different sources (i.e., wild-type and p75NTR-null mice). The vertical error bars indicate SEM (n = 5). D, Sortilin antagonist neurotensin abrogates proNT-3 induced SCG apoptosis. NGF-deprived rat SCG neuron cultures were treated with no ligand (None), 10 ng/ml NGF, or equal molar of NT-3 or proNT-3 (i.e., 2 ng/ml NT-3 or 4 ng/ml proNT-3) in the presence or absence of 20 μm neurotensin as indicated. Neuronal apoptosis under each culture condition was evaluated 48 h later, and the data were normalized to that of apoptotic neurons without any trophic factor or neurotensin treatment. Data represent the results obtained from three independently conducted experiments. The vertical error bars indicate SEM. E, Sortilin antiserum blocks apoptosis of proNT-3-treated SCG neurons. NGF-deprived rat SCG neuron cultures were treated with 10 ng/ml NGF or equal molar of NT-3 or proNT-3 (i.e., 2 ng/ml NT-3 or 4 ng/ml proNT-3) in the presence of an anti-sortilin antiserum (1:10 dilution) or the equivalent dilution of preimmune serum as a control (for characterization of the antiserum, see supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Neuronal apoptosis under each culture condition was evaluated 48 h later. The data represent the results obtained from three independently conducted experiments. The vertical error bars indicate SEM. Note that sortilin antiserum specifically inhibits proNT-3-induced cell killing without additional survival-promoting effects on NGF-deprived neurons. For C–E, asterisks denote statistical significance between the paired samples.