Figure 2. yHsp90 tyrosine phosphorylation is cell cycle associated, occurs in the nucleus, and marks Hsp90 for ubiquitination and proteasome-mediated degradation.
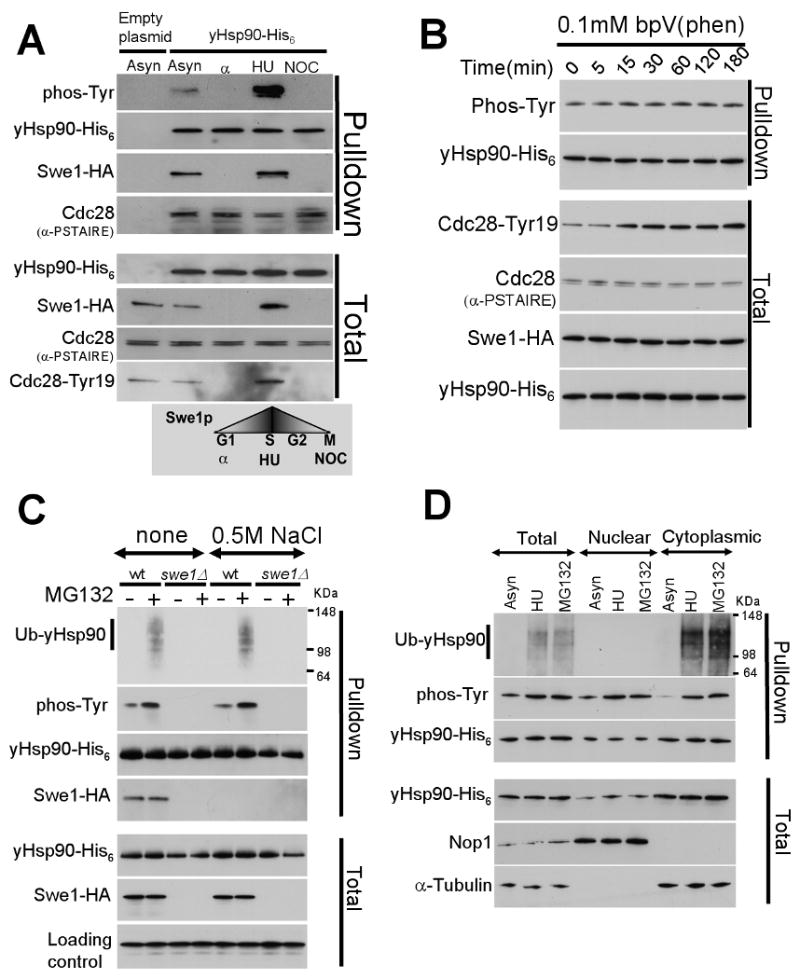
A) Yeast cells were arrested in G1 (with α-factor), in S-phase (with hydroxyurea, HU), and in M-phase (with nocodazole, NOC), and yHsp90 tyrosine phosphorylation was assessed. Cdc28 Tyr19 phosphorylation is shown as an indicator of Swe1 activity. Cdc28 interaction with yHsp90 was detected by α-PSTAIRE antibody.
B) Treatment of yeast with 0.1 mM bpV(Phen) did not increase tyrosine phosphorylation of yHsp90. Increase phosphorylation of Cdc28-Tyr19 was used as an indicator of phosphatase inhibition. Cdc28Cdc2 was detected by anti-PSTAIRE-antibody and Swe1-HA by anti-HA antibody.
C) Wild type or swe1Δ yeast cells expressing His6 tagged yHsp90 were treated with the proteasome inhibitor MG132 (50 μM for 1 h), and yHsp90 tyrosine phosphorylation and ubiquitination were assessed. Ub-yHsp90-His6 was detected using anti-ubiquitin antibody. The experiment was performed with and without salt stripping (0.5 M NaCl) of yHsp90 pulldowns.
D) Nuclear and cytoplasmic protein fractions were prepared from asynchronized (Asyn), S-phase arrested (HU), and MG132-treated cells. yHsp90-His6 pulldowns were blotted with anti-ubiquitin and anti-phosphotyrosine antibodies. Antibodies to Nop1 and α-tubulin were used to monitor purity of subcellular fractions.
