Figure 6. Co-chaperone binding to non-phosphorylatable Hsp90 mutants.
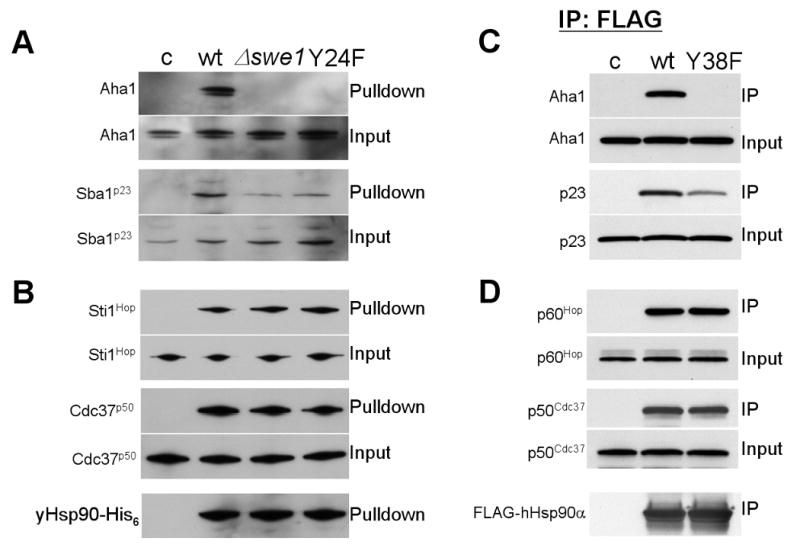
A) & B) yHsp90-His6 from wt or swe1Δ yeast and yHsp90His6-Y24F were precipitated and their interaction with co-chaperones was detected by immunoblotting.
C & D) COS7 cells were transfected with indicated FLAG-Hsp90 constructs (empty vector pcDNA3 [c], FLAG-hHsp90α [wt], or FLAG-hHsp90α-Y38F [Y38F]). FLAG-Hsp90 was immunoprecipitated and associated Aha1, p23, p60Hop and p50Cdc37 were detected by immunoblotting.
