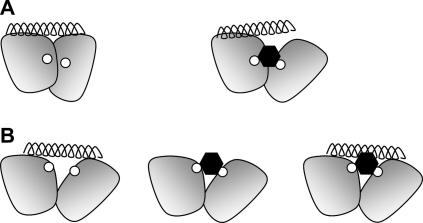
Fig. 3.
Two models for the mechanism of action of the antagonists BIBN4096BS and MK-0974. The N-terminal domains of CLR and RAMP1 are depicted by the two shaded shapes, the antagonist-binding residues Met-42 and Trp-74 are each shown as small white circles, the antagonist as a black hexagon and the peptide ligand as a helix. Both A(left) and B(left) depict how the peptide can interact with both domains but not with the antagonist-binding residues. In A(right) the antagonist binding at the CLR:RAMP1 interface causes an allosteric effect which alters the peptide-binding site. In B the antagonist binding (centre) results in the occupation of some of the space required for the binding of the peptide, leading to competitive binding (right).