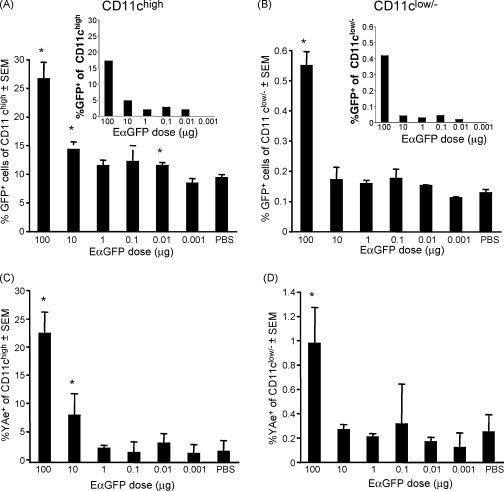
Fig. 2.
Dose-related detection of GFP-containing and pMHC+ cells in peripheral lymph nodes 24 h after Ag injection. Different doses of EαGFP Ag (100 μg, 1 μg, 0.1 μg, 0.01 μg, 0.001 μg) each containing 1 μg LPS were administered by subcutaneous injection in the neck scruff. Mice immunised with PBS containing 1 μg LPS were used for controls to establish the baseline staining and assay sensitivity. Draining proximal CLNs and BLNs were collected at 24 h from individual mice. The proportion of GFP+ cells in gated CD11chigh (A) and CD11clow/− (B) populations was determined for each sample by flow cytometry and results expressed as group mean percentage ± standard error of the mean (SEM). The high proportion of apparently GFP+ CD11c+ cells in the PBS/LPS control (∼10–15%) is due to background autofluorescence in the CD11c+ gate resulting from the collection of the large numbers of cells needed for analysis of the small fraction (1–4%) of lymph node CD11c+ cells. If the mean autofluorescence of the PBS control group is subtracted (insets in A and B) from the mean percentage of the groups receiving 0.001–100 μg EαGFP, it is clear that GFP+ cells are found in draining LNs for all antigen doses ranging from 100 μg to 10 ng. The proportion of CD11chigh and CD11clow/− cells displaying pMHC complexes is shown in (C) and (D), respectively. Mouse IgG2b was used as the Y-Ae isotype control for each sample and used to set positive and negative gates. Error bars show SEM for each group, where n = 3. Statistical comparisons (Students’ unpaired t-test, 2 way, assuming unequal variance) were made between the PBS control group and each dose and asterisk (*) indicates statistical significance where p < 0.05.