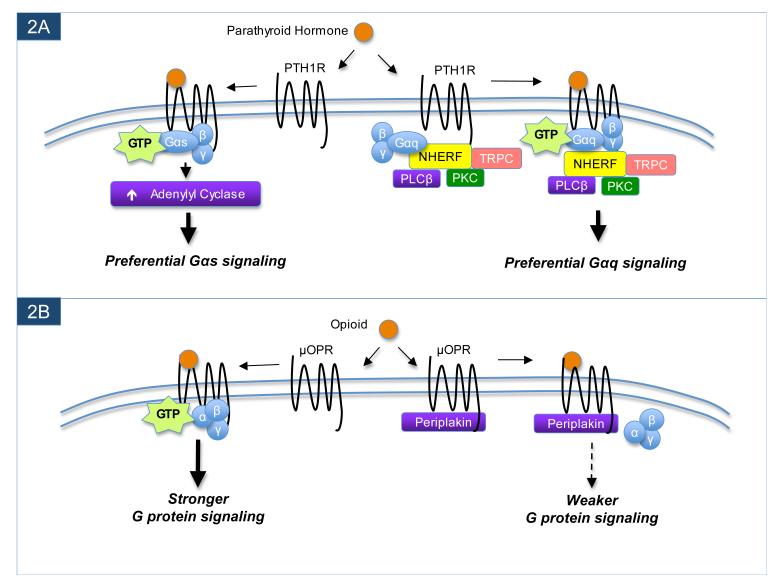
Figure 2.
GPCR-interacting proteins can modulate G protein-mediated signaling. In the example shown in panel A, interaction between the NHERF scaffold proteins and the parathyroid hormone receptor (PTH1R) leads to a preferential enhancement of the downstream Gαq-mediated signaling by PTH1R. Through its various protein-protein interaction domains, NHERF not only binds to PTH1R, but also tethers multiple downstream signaling effectors in close proximity to the PTH1R, thereby creating an efficient complex for Gαq-mediated signaling. However, when PTH1R is in a different cell type or separate cellular compartment in which NHERF proteins are absent, PTH1R instead preferentially signals through Gαs to activate adenylyl cyclase. A contrasting example is shown in panel B. Periplakin can associate with the μ opioid receptor (μOPR) to attenuate G protein-mediated signaling. However, in cell types or cellular compartments where periplakin is not found, μOPR ligands can more robustly activate the receptor to stimulate signaling by G proteins.