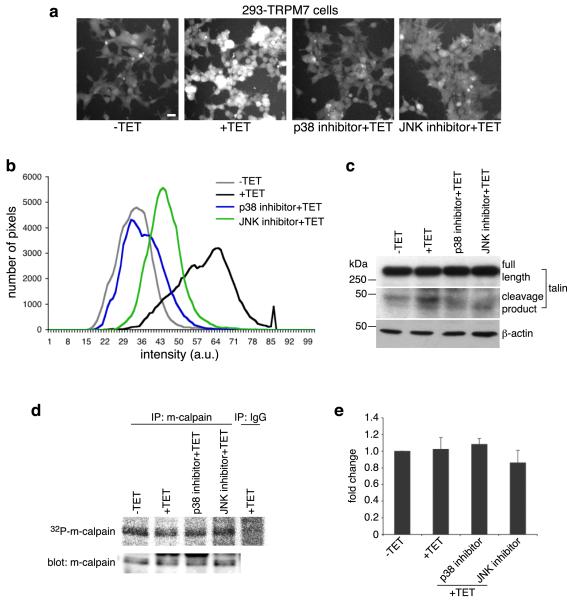
Figure 2.
p38 MAPK and JNK are required to stimulate m-calpain activity when TRPM7 is overexpressed. (a) Calpain activity assay using fluorogenic BOC-LM-CMAC substrates. Overexpression of TRPM7 (+TET) stimulated intracellular cleavage of the synthetic calpain substrate producing greater cellular fluorescence emission compared to control cells (−TET). Treatment of 293-TRPM7 expressing cells with p38 MAPK inhibitor SB203580 and JNK inhibitor SP600125 inhibited the TRPM7-dependent increase in fluorescence emission. Scale bar is equal to 100 μm. The experiment was repeated three times with similar results. (b) The intensity of fluorescence emission from cells under the conditions depicted in (a) was quantified and displayed as a histogram of the distribution of intensity. The x-axis represents fluorescence emission intensity and the y-axis indicates the number of pixels found for each respective intensity. (c) Western blots using a monoclonal antibody that recognizes the head domain of talin was used to probe cell lysates from 293-TRPM7 cells treated with tetracycline in the presence of various inhibitors. Expression of TRPM7 caused an increase in the cleavage of m-calpain substrate talin into its head and rod domains. The head domain cleavage product migrated at 47 kDa. Application of p38 MAPK inhibitor SB203580 and JNK inhibitor SP600125 reduced proteolysis of talin in response to TRPM7 expression. A western blot of β-actin is shown to demonstrate equal loading of samples. (d) In vivo [32P]orthophosphate labeling of 293-TRPM7 cells treated with tetracycline in the presence of various inhibitors. Shown is an autoradiograph of incorporation of [32P] into m-calpain immunoprecipitated from cell lysates. An anti-m-calpain blot is shown to demonstrate the quantity of immunoprecipitated protein. (e) Quantification of [32P]orthophosphate incorporation normalized to m-calpain protein. Results are presented as fold change compared to the negative control (−TET). Values are mean ± standard deviation of five independent experiments.