Abstract
Background
Human monocytes play an important role in mediating human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system (CNS), and monocytes-derived macrophages (MDM) represent a major viral reservoir within the brain and other target organs. Current gene transduction of MDM is hindered by a limited efficiency. In this study we established a lentiviral vector-based technique for improved gene transfer into human MDM cultures in vitro and demonstrated significant protection of transduced MDM from super-infection with wild-type HIV-1.
Methods
HIV-1-based lentiviral vector stocks were prepared in 293T cells by the established calcium phosphate transfection method. Human monocytes were isolated from donors' blood by Ficoll-Paque separation and cultured in vitro. To establish an effective technique for vector-mediated gene transfer, primary cultures of human MDM were transduced at varying multiplicities of infection (MOI) and at a range of time points following initial isolation of cells (time-in-culture). Transduced cells were then examined for transgene (green fluorescent protein (GFP)) expression by fluorescent microscopy and reverse transcription polymerase chain reaction (RT-PCR). These cultures were then exposed to wild-type HIV-1, and viral replication was quantitated by p24 assay; production of neurotoxic effector molecules by the transduced MDM was also examined, using indicator neurons.
Results
We have demonstrated that primary human MDM could be efficiently transduced (>50%) with concentrated HIV-1-based defective lentiviral vectors (DLV). Furthermore, DLV-mediated gene transduction was stable, and the transduced cells exhibited no apparent difference from normal MDM in terms of their morphology, viability and neurotoxin secretion. Challenge of DLV-transduced MDM cultures with HIV-1Ba-L revealed a 4- to 5-fold reduction in viral replication, as measured by p24 antigen production. This effect was associated with the mobilization of the GFP-expressing DLV construct by the wild-type virus.
Conclusions
These data demonstrate the inhibition of HIV-1 replication in primary MDM, by a DLV vector that lacks any anti-HIV-1 transgene. These findings lay the initial groundwork for future studies on the ability of DLV-modified monocytes to introduce anti-HIV-1 genes into the CNS. Lentiviral vector-mediated gene delivery to the CNS by monocytes/macrophages is a promising, emerging strategy for treating neuro-AIDS.
Keywords: defective lentiviral vector (DLV), monocots-derived macrophages (MDM), blood-derived monocytes (BDM), HIV-1, transduction efficiency, vector mobilization (VM), green fluorescent protein (GFP)
Introduction
HIV-1-based vectors are attractive gene delivery tools because of their ability to efficiently transduce target cells independent of their cell division status at both dividing and non-dividing phases, and their capacity to establish long-lasting transgene expression due to chromosomal integration of the proviral DNA. Among the cell types which are susceptible to HIV-1-based vectors are hematopoietic stem cells [1–7] and monocytes/macrophages [8–13] which represent important targets for human gene therapy [14–16].
Monocytes arise in the bone marrow from myeloid stem cells and are released into the blood. Within a short time, they migrate into various tissues and mature into macrophages. These mononuclear cells also have the ability to cross the blood-brain barrier (BBB) and to enter the central nervous system (CNS), where they act as an important target and reservoir of HIV-1 [17–21]. HIV-1-infection of the brain can lead to HIV dementia, a primary disorder of the CNS, which affects nearly 25% of untreated, HIV-1-infected individuals [22]. Highly active antiretroviral therapy (HAART) has failed to eliminate this aspect of HIV-1-associated disease, and in fact the prevalence of HIV dementia has increased in the post-HAART era, possibly because of HAART's positive effects on overall patient survival time [23–25]. It has further been suggested that HAART may be altering the presentation and/or pathogenesis of HIV dementia, resulting in a more slowly progressive, chronic form of the disease [23–25]. These observations highlight the need for new, adjunct therapeutic approaches aimed specifically at ameliorating or preventing HIV dementia.
One exciting approach is to genetically modify MDM, so as to render them resistant to HIV-1 infection. An additional merit of this approach is that blood-derived monocytes (BDM) are able to traverse the BBB, and to differentiate into stable, long-lived, CNS-resident macrophages; this suggests that BDM may represent a potential vehicle to introduce anti-HIV-1 or neuroprotective genes into the brain. Partly because of this, there is a growing interest in the development of efficient methods for gene transfer into primary human monocyte-derived macrophages (MDM) and for manipulation of gene expression in these cells. Several groups have attempted to transduce MDM recently and different viral vectors have been tested for their effectiveness at transducing monocytes and macrophages [8,26–29]. However, only limited success has been obtained to date. One of the major obstacles in delivering therapeutic genes into human MDM is the poor transduction efficiency under most currently available gene-transfer conditions [8,26,28–31].
We have previously described the construction of defective lentiviral vectors (DLV) based on a modified HIV-1 genome [13,32]. We have used these vectors to develop an optimized protocol for the efficient transduction of primary human MDM. We explored the effect of a range of different parameters on the efficiency of DLV-mediated gene transfer into these cells, including cell density, time-in-culture, and the multiplicity of infection (MOI) of the vector. By doing so, we developed an optimized protocol, which allowed us to transduce approximately 50% of our target cell population. These transduced cells showed stable, long-term expression of the green fluorescent protein (GFP) and exhibited no apparent difference from untransduced normal MDM in terms of cell morphology and growth kinetics. When challenged with an infectious wild-type HIV-1Ba-L strain, the DLV-transduced cultures showed a significant ability to inhibit HIV-1 replication, as evidenced by a roughly 5-fold reduction in p24 levels in the transduced MDM compared to untransduced normal MDM. These results represent the initial report of inhibition of wild-type HIV-1 replication in human MDM by a DLV vector that lacks any anti-HIV-1 transgene, suggesting the possibility that DLV-modified MDM may represent a potentially useful approach for anti-HIV-1 therapy.
Materials and methods
Vector production and concentration
HIV-1-based defective lentiviral vectors (DLV) encoding selected cis-acting elements were produced by transient transfection of human embryonic kidney 293T cells with a packaging construct, a VSV-G envelope construct and a transfer construct containing the reporter gene GFP (Figure 1) [13,33]. In brief, 24 h prior to transfection, 293T cells were seeded in 75 cm2 tissue culture (TC) flasks at a density of about 6.5 × 106 cells/flask with 14 ml Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and incubated at 37 °C with 5% CO2. Cell growth medium was replaced with 14 ml fresh medium 2 h prior to transfection. For transfection, a mixture of 6.25 μg packaging plasmid, 6.25 μg transfer plasmid and 2.50 μg VSV-G encoding envelope plasmid was added dropwise to each 75 cm2 TC flask, and then chloroquine was added to a final concentration of 25 μM. Eight hours post-transfection, the medium was aspirated from these flasks and 8 ml/flask of fresh DMEM containing 2% FBS was added. Vector produced in the transfected cultures was harvested at days 1,2, and 3 post-transfection and stored at −70 °C. Titration of vector was performed using a human T cell line, CEM (AIDS Research and Reference Reagent Program, NIH), by a 10-fold serial dilution method and vector titer was determined at day 3 post-inoculation by counting the number of GFP-positive cells at the endpoint of vector dilution using a fluorescence microscope.
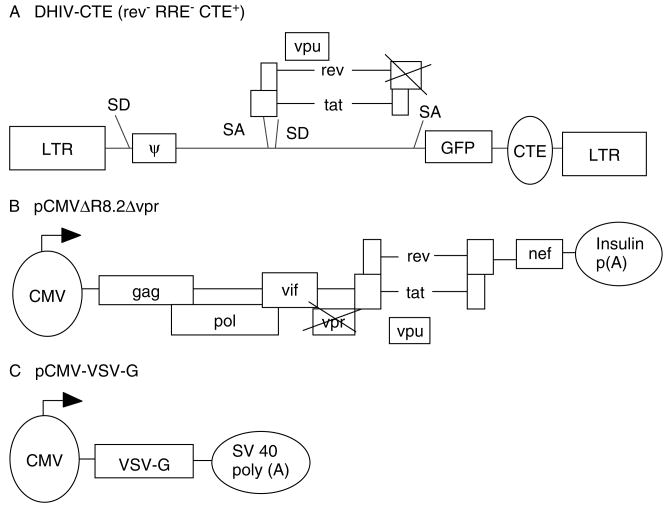
Figure 1.
Generation and design of defective lentiviral vector (DLV). (A) HIV-1-based transfer construct lacking structural genes (gag/pol and env) and most accessory genes (vif, vpr, nef and rev) (32). (B) pCMVΔR8.2Δvpr is the lentiviral packaging construct [42]. (C) The envelope construct, pCMV-VSV-G, encodes the vesicular stomatitis virus glycoprotein (G) for pseudo-typing [1]
To obtain highly concentrated DLV stocks, vectors stored at −70 °C from different batches of viral transfection were thawed and pooled. Following low-speed centrifugation (1800 g) for 30 min at 4°C, recovered supernatant was passed through a 0.45 μm filter (Nalgene, USA). Filtrate containing the vector was then ultra-centrifuged at 113 000 g for 3 h at 4°C using a Beckman SW28 rotor. Vector pellet was resuspended in a small amount (1% original volume) of RPMI-1640 containing no FBS. Vector aliquots (0.1–0.2 ml/tube) were stored at −70 °C for future use.
In vitro isolation and cultivation of monocytes
Blood was drawn from consented healthy donors into BD Vacutainer™ ACD (Beckton Dickinson), and peripheral blood mononuclear cells (PBMC) were isolated by density-gradient centrifugation through Ficoll-Paque™ PLUS (Amershan Biosciences AB, Sweden). The purified PBMC were then resuspended in RPMI-1640 medium (Sigma) supplemented with 10% heat-inactivated human serum (Gemini Bio-Products, CA, USA), 20% heat-inactivated FBS (Hyclone), 100 U/ml penicillin, 100 μg/ml streptomycin sulfate and 0.292 mg/ml L-glutamine (Sigma). The purified PBMC were then seeded into 25 cm2 TC flasks (Corning) at a range of different densities (0.3 to 3.0 × 107 cells) and incubated at 37 °C with 5% CO2 to allow the attachment of freshly isolated monocytes. Following 3 h incubation, non-adherent cells were removed by aspiration and the remaining adherent cells were washed extensively with Dulbecco's phosphate-buffered saline (DPBS) (Sigma), prior to the addition of 5 ml RPMI-1640 growth medium per flask, and subsequent cultivation at 37 °C. Cell growth and monolayer formation were observed daily using a phase-contrast inverted microscope (Olympus).
To verify the purity of the attached cells, these monolayer cultures were stained with human CD14 monoclonal antibody conjugated with R-phycoerytherin (Caltag Laboratories, CA, USA). Briefly, the antibody was diluted 50–100 times with DPBS and MDM cultures at incubation days 1, 4, 7 and 11 were stained with the diluted antibody. After 1–2 h incubation, stained cells were rinsed twice with DPBS, and then examined under an inverted fluorescence microscope (Olympus IX70). Mouse isotype IgG2a conjugated with R-PE was also included in this study as a negative control.
Transduction of primary human MDM
Before DLV transduction, cell growth medium was removed from flasks and MDM cultures were washed gently with DPBS three times to remove all cellular debris and residual serum. Transduction was initiated by adding 0.5 ml of DLV stock to each 25 cm2 TC flask, in the presence of 8 μg/ml polybrene (Sigma). Following a 2-h adsorption with gentle shaking every 15–20 min, transduced cells were rinsed twice with DPBS to remove residual vectors and then 5 ml of complete RPMI-1640 growth medium were added to each flask. The cultures were subsequently evaluated for the expression of the transgene (GFP) by visual inspection using an inverted phase-contrast fluorescence microscope. Efficiency of DLV-mediated transduction of primary MDM was determined at day 3 post-transduction by calculating the percentage of GFP-positive cells within the transduced cell population.
This transduction protocol was used to test and evaluate freshly isolated monocytes (day 0) and primary MDM cultivated for different time periods in vitro (days 3, 5, 7, 9 and 13) for their sensitivity to DLV infection at an MOI of 10. In some experiments, the efficiency of DLV-mediated transduction was also examined using a range of different concentrations of vector MOI (1, 10, 50 and 100), and the effect of multiple rounds of vector transduction was also determined.
Neurotoxin production by DLV-transduced MDM
For analysis of the in vitro neurotoxicity of MDM-derived supernatants, we followed our previously described methods [34,35]. In brief, cell culture conditioned medium was collected from DLV-transduced MDM at days 0, 5 and 10 days post-infection and stored at −70 °C until analysis. Cell culture supernatants were also collected from untransduced normal MDM and HIV-1Ba-L-infected MDM cultures as controls. To determine neurotoxin production, conditioned medium collected from DLV-infected MDM and control MDM cultures was diluted at 1:10 and then applied to primary neuronal cells prepared from the cerebella of postnatal day 8 Sprague-Dawley rats as previously described [36–38]. Following 24-h incubation, cells were washed and fixed with 4% paraformaldehyde in 0.1 M PBS, and then subjected to an in situ terminal deoxynucleotidyl transferase-mediated digoxigenin-dUTP nick end-labeling (TUNEL) assay (Oncor, Gaithersburg, MD, USA) as described previously [35]. Determination of neurotoxin production by transduced MDM was performed by measuring the percentage of apoptotic neurons from 12 or more microscopic fields of TUNEL-stained cells.
Wild-type HIV-1 infection of DLV-transduced MDM
HIV-1Ba-L, obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, was propagated once in primary MDM in order to generate a cell-free virus stock, which was then used to infect primary MDM. For this experiment, a DLV-transduced cell population containing approximately 45 ± 3% GFP-positive cells, and a mock-transduced control population, was challenged with wild-type HIV-1Ba-L (40 ng of p24 antigen) at day 5 post-transduction. Following 1 h adsorption at 37 °C, the virus was aspirated from the flasks and the cells were washed twice with DPBS (5 ml/wash), prior to the addition of growth medium. Culture supernatants were collected every 3 days for a total period of 4 weeks, and HIV-1 production in these infected cultures was determined by measuring the level of HIV-1 p24 antigen in these supernatants.
Statistical analysis
Both one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test (software ‘Prism’) and t-test were employed for statistical analysis in this study. The difference between two samples was considered to be significant if the p value was less than 0.05.
Results
Primary cultures of human MDM
To optimize an in vitro method for consistent preparations of primary cultures of human MDM for vector-based genetic modification, we evaluated the effect of initial cell-seeding density and cell attachment time on cell growth and longevity in vitro. We found that an 80–90% confluent cell monolayer was usually formed within 3 days of seeding 1.5 ± 0.5 × 107 PBMC into a 25 cm2 TC flask. Staining of the adherent cells with a mouse anti-human CD14 monoclonal antibody showed that 94–98% of the cells were CD14-positive (Figure 2), indicating the purity of prepared monocyte cultures.
Figure 2.
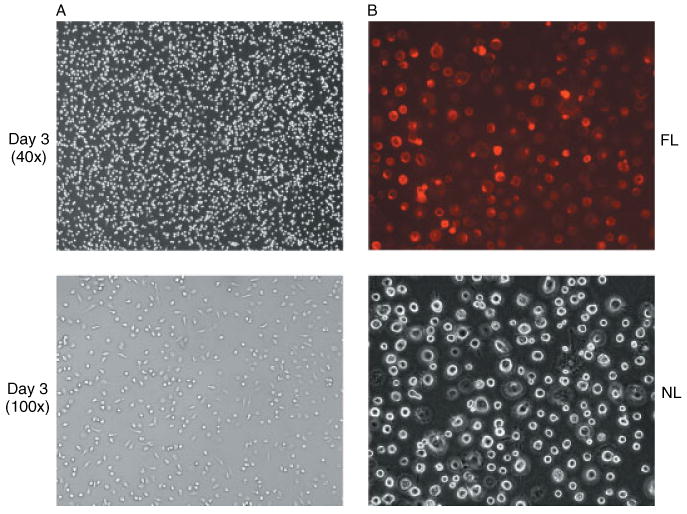
Photomicrographs of primary cultures of human MDM. (A) Day 3 cultures of human MDM showing the formation of cell monolayers under phase-contrast microscopy. (B) Staining of primary cultures of human MDM at day 10 with mouse anti-human CD14 monoclonal antibody conjugated with R-phycoerytherin showing the same field under either normal light (NL) phase-contrast microscopy or fluorescent light (FL) microscopy (magnification 200×)
Transduction of primary MDM
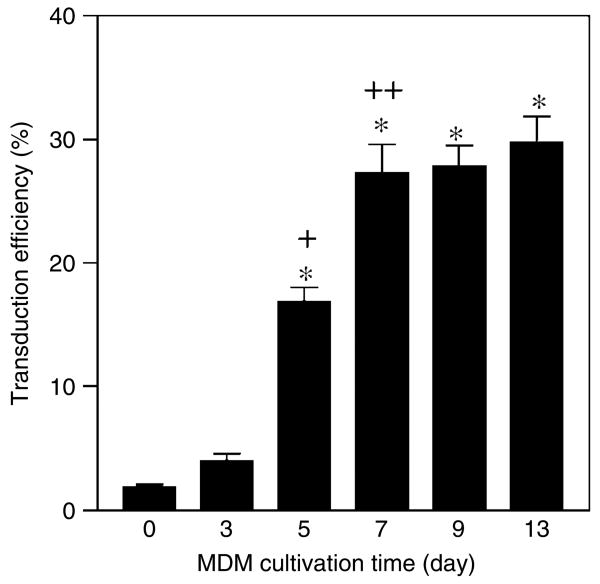
We tested DLV for their ability to transduce cultures of primary human monocytes that had been maintained in vitro for different time periods (0, 3, 5, 7, 9 and 13 days). Freshly isolated monocytes (day 0) were found to be susceptible to DLV transduction at an MOI of 10, but at a very low efficiency (1.9 ± 0.4%; Figure 3). However, the cells became progressively more susceptible to DLV-mediated transduction during their first week in culture. DLV-directed transduction efficiency of MDM increased to over 4% at day 3 and reached approximately 17% at day 5 (p < 0.001). When day 7 cultures were used, over 27% of the cell population was successfully transduced at the vector MOI of 10. However, as indicated in Figure 1, the transduction efficiency did not significantly change after day 7 (p > 0.05). These results suggest that DLV-mediated gene transfer into primary MDM may be influenced by cellular differentiation or by other factors associated with in vitro maintenance of primary monocytes [8,39].
Figure 3.
Effect of time-in-culture on the susceptibility of primary human monocytes to DLV transduction. Adherent monocytes were grown in RPMI supplemented with 10% human serum and 20% FBS and transduced with DLV (MOI = 10) at the indicated times. Transduction efficiency was determined by calculating the percentage of GFP+ cells within the culture. Transduction efficiency increased significantly from day 3 to day 5 (+p < 0.001) and from day 5 to day 7 (++p < 0.01), but there was no significant increase after day 7 (*p > 0.05) using one-way ANOVA followed by Bonferroni's multiple comparison test
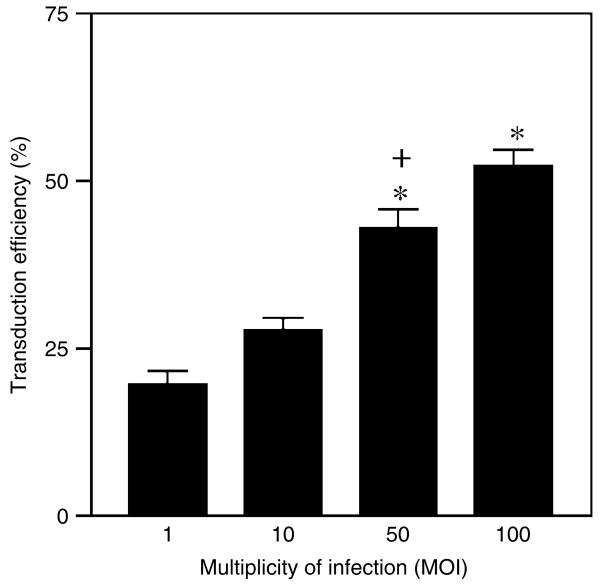
We also evaluated the impact of vector concentration on transduction efficiency of primary MDM. For this experiment, primary MDM cultures on day 7 in vitro (DIV-7) were exposed to 1, 10, 50, and 100 MOI of the DLV. We demonstrated that the DLV-mediated transduction efficiency was directly correlated with the vector MOI that was used (Figure 4), although not in a linear fashion (i.e., gene transfer efficiency was elevated only about 2.5-fold, when going from an initial MOI of 1.0 to an MOI of 100). Nonetheless, we were able to attain a high efficiency of the gene transfer at the highest MOI (52% of cells in the culture being GFP-positive at this MOI), indicating a statistically significant increase of DLV-mediated gene transduction (p < 0.001).
Figure 4.
Effect of different vector MOI on transduction efficiency in human monocyte cultures. MDM cultures (day 7 in vitro) were seeded at a common density and then transduced with the indicated vector MOI. The percentage GFP+ cells within the culture was then determined at day 5 post-transduction. Data are given as the mean of multiple samples from at least three independent experiments for each cell culture time point. Transduction efficiency increased significantly when a MOI of 50 or 100 was used as compared to MOI of 1 (*p < 0.005) and 10 (+p < 0.01) by one-way ANOVA followed by Bonferroni's multiple comparison test
To further enhance DLV-mediated gene transfer into primary MDM, we tested and established a supertransduction protocol. We hypothesized that initial efficiency of DLV-mediated transduction of human MDM would be significantly elevated by repeated vector transduction, i.e., by re-transducing a previously transduced MDM population with DLV. As summarized in Table 1, initial transduction efficiency could be substantially increased by multiple rounds of subsequent transduction.
Table 1.
Effect of DLV-mediated supertransduction on gene transfer efficiency in monocyte-derived macrophages
| Primary transduction | Second transduction | Third transduction | ||||||
|---|---|---|---|---|---|---|---|---|
| MOI | Efficiency | Mean ± SD | MOI | Efficiency | Mean ± SD | MOI | Efficiency | Mean ± SD |
| 10 | 30.67% (46/150) | 10 | 43.00% (56/130) | 10 | 46.23% (49/106) | |||
| 24.85% (42/169) | 27.83 ± 2.91% | 33.50% (57/170) | 38.10 ± 4.76% | 53.66% (66/123) | 50.26 ± 3.75 | |||
| 27.96% (52/186) | 37.08% (66/178) | 50.89% (57/112) | ||||||
| 50 | 43.41% (73/150) | 54.87% (62/113) | ||||||
| 38.10% (56/147) | 43.02 ± 4.74% | 10 | 48.80% (61/125) | 52.67 ± 3.36% | ND | ND | ||
| 47.56% (78/164) | 54.35% (50/92) | |||||||
MOI = multiplicity of infection, SD = standard deviation
Both transduced and super-transduced primary MDM were analyzed for their morphology and growth kinetics compared to untransduced control cells; we found that these transduced cells exhibited no apparent difference from normal MDM. Consistent with this, we observed that the expression of the GFP reporter gene in transduced MDM remained stable for >7 weeks in culture by phase-contrast microscopy (Figure 5A) and by reverse transcription polymerase chain reaction (RT-PCR) (Figure 5B).
Figure 5.
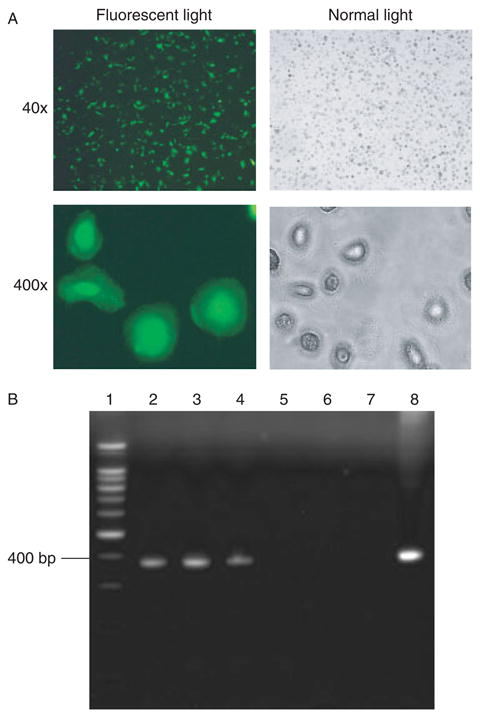
Stable gene expression in human MDM. (A) Primary cultures of human MDM were transduced with the defective lentiviral vector (MOI = 50) at day 7 in vitro, and GFP expression was then examined 46 days later (one of four representative experiments is shown). (B). RT-PCR detection of transgene expression in DLV-transduced human MDM. Total RNA was isolated from both DLV-transduced and normal MDM and RT-PCR amplification was conducted with the use of GFP-specific primers flanking a 373-bp fragment. The PCR products were subjected to 2% agarose gel electrophoresis. Lanes: 1 = 100 bp DNA ladder; 2 = DLV-transduced MDM at day 5; 3 = DLV-transduced MDM at day 39; 4 = DLV-transduced MDM infected with HIV-1Ba-L at day 25; 5 = untransduced normal MDM at day 25; 6 = negative control (water) for RT; 7 = negative control (water) for PCR; and 8 = positive control, pDLV
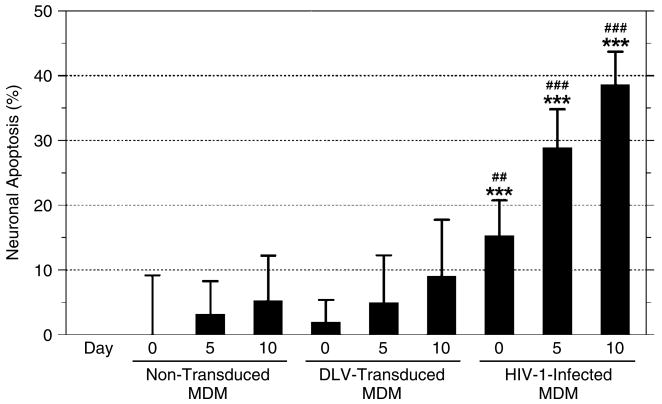
To evaluate whether vector transduction might result in cellular activation and release of potentially neurotoxic effector molecules, we also examined the in vitro neurotoxicity of supernatants collected from normal non-transduced MDM and MDM cultures infected with DLV or wild-type HIV-1Ba-L. This comparative analysis revealed that culture supernatants from DLV-transduced MDM elicited identical, baseline, levels of toxicity in indicator rat neurons, as compared to supernatants from normal untransduced MDM (Figure 6). In contrast, supernatants from HIV-1-infected MDM elicited significant levels of neuronal apoptosis, as expected [40].
Figure 6.
Macrophage conditioned medium (MCM) collected at various days post-infection (0, 5 and 10 days) from non-transduced, DLV-transduced, and HIV-1-infected MDM was added (at a dilution 1 : 10) into cultures of rodent cerebellar granule neurons for 24 h. At the end of this incubation period, neuronal apoptosis was evaluated by TUNEL assay. The data are presented as mean values (% apoptotic cells) ± the standard error of the mean. All values shown represent treatment-specific values (i.e., the basal level of apoptosis in untreated cells has been subtracted from these data). Statistical significance was determined by one-way ANOVA followed by Bonferroni's multiple comparison test. ***, ##, and ### denote statistical significance p < 0.001 as compared to non-transduced MCM on the corresponding day, p < 0.01 and p < 0.001 as compared to DLV-transduced MCM on the corresponding day, respectively
DLV-transduced cultures resist challenge with infectious HIV-1
To determine if DLV-mediated transduction of MDM could alter cellular susceptibility to infection with full-length HIV-1, we performed a challenge experiment in which we exposed both transduced and untransduced MDM to HIV-1Ba-L. As shown in Figure 7, HIV-1Ba-L replication was detected in the control MDM shortly after viral inoculation (day 3) and gradually reached peak levels by day 21 post-infection. On the other hand, HIV-1Ba-L replication in DLV-transduced MDM was significantly inhibited. As shown in Figure 7A, initial detection of viral replication was delayed and peak levels of virus production were suppressed by 4- to 5-fold, as compared to the non-transduced cultures. These results suggest that the HIV-1-based lentiviral vector conferred a considerable degree of protection against wild-type HIV-1 challenge in primary human MDM. In agreement with this, an HIV-1-induced cytopathic effect (CPE) in untransduced MDM was evident by the presence of abnormally large cells, multinucleated cells, and debris resulting from late stages of cell death. However, we observed only very modest levels of viral-induced CPE in the DLV-transduced cultures, as compared to control cultures (Figure 7B).
Figure 7.
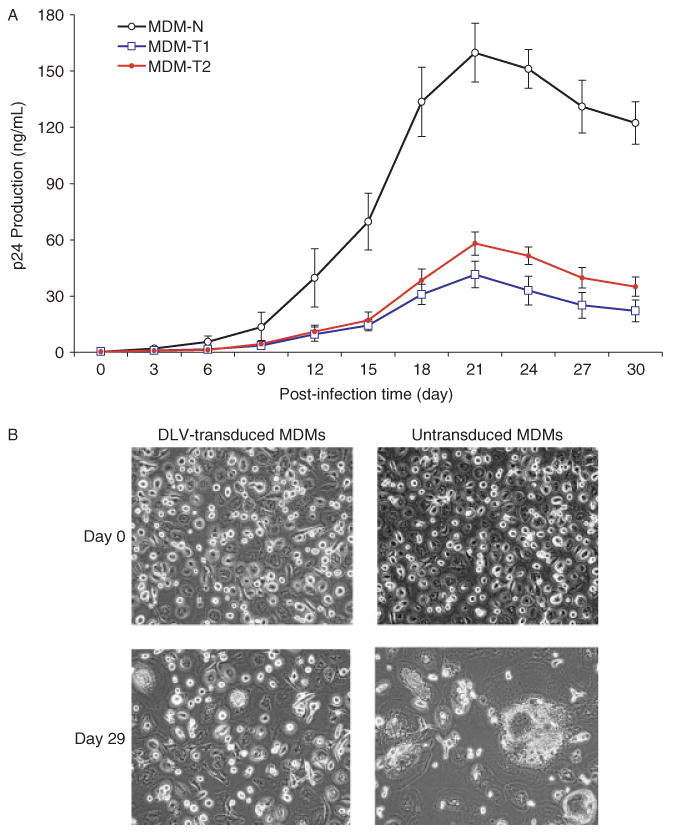
Lentiviral vector inhibits HIV-1 replication in primary monocyte cultures. (A) A comparison of the kinetics of HIV-1Ba−L replications shown, in both normal (non-transduced; MDM-N) and vector-transduced (MDM-T1 = DHIV-CTE transduced and MDM-T2 = DHIV-CTEΔvpu transduced) MDM. The data show a significant inhibition of HIV-1 replication in both the vector-transduced MDM cultures as compared to untransduced MDM (t-test, p < 0.01), but no statistical difference between MDM-T1 and MDM-T2 (p > 0.05). (B) DLV vector transduction suppresses HIV-1 cytopathicity in MDM cultures. Normal (non-transduced) and DHIV-CTE-transduced cultures of primary MDM were exposed to HIV-1Ba−L, and examined under light microscopy at days 0 and 29 following virus infection (100× magnification). It can be readily appreciated that the DLV strongly suppressed HIV-1-mediated cytopathic effects, resulting in a reduction in the number of giant cells in the culture
The DHIV-CTE vector used in our experiments contains an intact vpu gene that is located in an intron. This could, in theory, be expressed upon HIV-1 infection of DLV-transduced target cells and might interfere with wild-type virus replication due to effects on CD4 receptor expression, or for other reasons. With this in mind, we modified DHIV-CTE by restriction enzyme-mediated ablation of the vpu gene to generate DHIV-CTEΔvpu. Infection with DHIV-CTEΔvpu displayed similar transduction efficiency as the parental DHIV-CTE (data not shown). We then performed a challenge experiment, in which DHIV-CTEΔvpu-transduced MDM cultures containing 44 ± 2% GFP-positive cells were exposed to wild-type HIV-1Ba-L under the same conditions as used in the experiment shown in Figure 7. The results showed a substantial reduction (67 ± 3%) in HIV-1Ba-L replication as compared with untransduced control MDM by measuring p24 antigen production at days 18–24 post-infection (t-test, p < 0.01). The extent of the anti-HIV-1 effect was very slightly lower than that in the DHIV-CTE-transduced cultures (76 ± 2% viral reduction; Figure 7A), but the two data sets did not differ to a statistically significant degree (t-test, p > 0.05). These findings suggest that the HIV vpu gene product may contribute to the DLV-mediated inhibition of wild-type HIV-1 replication at a very limited level in our experimental system.
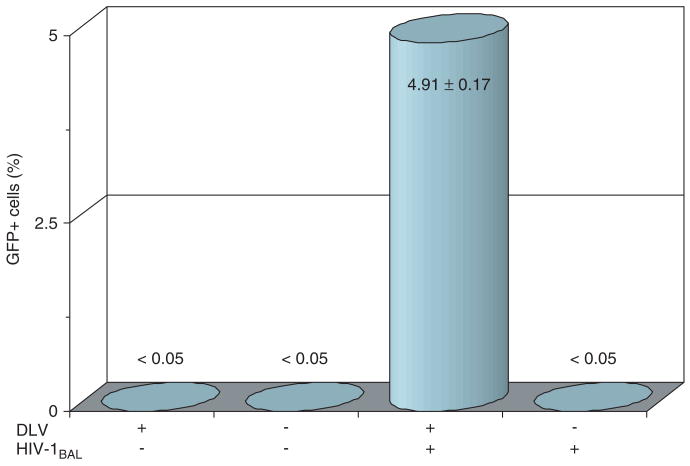
Finally, to evaluate DLV mobilization, we examined the percentage of GFP-positive cells in the virus-challenged cell cultures described above. This analysis revealed that the percentage of GFP-positive cells increased from an initial level of 44–45% at the time of infection, to a level of 55–57% at day 30 following infectious virus challenge, in both the DHIV-CTE- and DHIV-CTEΔvpu-transduced MDM cell cultures. This change could be attributed to (a) vector mobilization which might have occurred within the culture system and/or (b) preferential killing of untransduced (GFP-negative) cells. Our experimental results do not provide enough information to allow us to infer whether (a) or (b) was more prominent. To examine the former possibility, culture supernatants were collected from the DHIV-CTE-transduced MDM at 15 days post infection with HIV-1−Ba−L. After performing low-speed centrifugation (1800 g, 30 min) and membrane filtration (0.45 μm filter), the resulting cell-free supernatants were then used to infect freshly prepared monocytes at day 7. This resulted in the detection of some GFP-positive cells as early as post-infection day 2 (0.5 ± 0.1%) which reached 4.9 ± 0.17% within the infected MDM population at day 6, consistent with the mobilization of the vector by HIV-1Ba-L (no mobilization was detected in cultures that were not exposed to HIV-1Ba-L, nor was any mobilization detected in supernatants from untransduced MDM that were exposed to HIV-1Ba-L; Figure 8). Importantly, these newly infected monocyte cultures remained GFP-positive over an extended time period (12 days) and showed no alteration in the number of GFP-positive cells during the extent of the experiment, indicating that pseudotransduction or transcription from unintegrated virus was not responsible for the detection of GFP.
Figure 8.
DLV mobilization by wild-type HIV-1 infection. Mobilization of the GFP-expressing DLV vector was assessed by collecting cell-free culture supernatants from the indicated cell populations. These culture supernatants were then added to fresh MDM and the percentage of GFP-positive cells was enumerated 3 days later. Results shown represent mean values from three independent experiments (± the standard deviation). The data clearly show that DLV mobilization was induced in transduced MDM when they were subsequently infected with HIV-1Ba-L, but not in transduced but uninfected cells, or in untransduced MDM
Discussion
In this paper, we describe a reproducible and simple method for the highly efficient transduction of primary human monocytes and MDM. This method results in transgene expression among >50% of the transduced cell population, and therefore represents a major improvement over previously published methods [8]. It may be possible to further improve the efficiency of gene transfer by incorporating the cPPT element into our vector backbone, as described by Neil and coworkers [8].
Our results demonstrate that the efficient transduction of primary human monocytes with HIV-1-based vectors is dependent, in part, on the in vitro cultivation of these cells for a period of approximately 7 days (at least under our cell culture conditions). This is in good agreement with results from other investigators, and reinforces the notion that freshly isolated human monocytes are somewhat resistant to HIV-1 infection [8], for reasons which may relate to a block in nuclear import of the viral pre-integration complex [8], and/or deficiencies in cellular cofactors that are required for efficient viral reverse transcription [39].
Importantly, DLV-mediated transduction of primary monocytes did not result in changes in cell growth properties or in the production of neurotoxic effector molecules – suggesting that it had only a minimal impact on cellular activation. At the same time, transgene expression in transduced MDM was very stable in vitro. Collectively, these findings bode well for the potential future use of lentivirus-transduced monocytes in an in vivo setting (e.g., to act as ‘Trojan horses’ to deliver transgenes into the CNS, for long-term expression within the CNS).
Because human MDM are major targets and reservoirs of HIV-1 within the CNS [17,20], we wished to determine whether DLV-transduced MDM might be partially or completely resistant to infection by wild-type HIV-1. We therefore examined the susceptibility of untransduced MDM and matched, transduced MDM to infection by HIV-1Ba-L. We found that the replication of HIV-1 in the transduced MDM was significantly inhibited when compared to untransduced normal MDM. These data demonstrate a DLV-mediated inhibitory effect on HIV-1 infection in primary culture of human MDM (using vectors which lack any designated anti-HIV-1 insert sequence), which extend our previous findings in dividing cells [32].
DLV-mediated interference of HIV-1 replication is a recently described phenomenon that has been demonstrated in transduced primary lymphocytes and CD4+ T cell lines by several other investigators [32,41–47]. There are several potential mechanisms that may account for this antiviral effect: (a) TAR and RRE decoy effects of the vectors at both the transcriptional and post-transcriptional levels [45]; (b) competition of the vectors for substrates necessary for reverse transcription and RNA encapsidation [44,46]; and (c) co-packaging and/or dimerization of wild-type and DLV genomes, resulting in the generation of defective virus particles [48]. Precedents for some or all of these possibilities exist in the literature, and include the fact that co-packaging of wild-type HIV genome along with a modified HIV genome and subsequent inhibition of HIV-1 replication has been documented using a Moloney retrovirus vector [49]. The present study suggests that these mechanisms of HIV-1 suppression hold true not only in T cell lines or primary CD4+ T cells, but also in primary human MDM.
Previous studies have shown that defective HIV-1-based vectors can be mobilized by wild-type HIV-1, and subsequently trafficked to previously untransduced cells within a culture [32,41,44–46]. This phenomenon remains relatively poorly explored, but its existence suggests that it may be possible to design a gene therapy strategy in which one might be able to protect a large number of virus-susceptible cells by transducing a much smaller initial subset of cells (either ex vivo or in vivo). In our experiments, we demonstrated that DLV mobilization can occur in transduced human monocytes, although the efficiency of vector mobilization appeared to be modest. We are currently examining whether it may be possible to increase the efficiency of mobilization.
It should be noted that the defective lentiviral vector (DHIV-CTE) used in this study contains an intact HIV-1 tat gene. It has been documented in the literature that the presence or absence of Tat has little or no effect on vector production or on the efficiency of vector transduction [44]. However, deletion of the tat gene from our vector would prevent detection of the GFP reporter gene, which is under the transcriptional control of the Tat-dependent HIV-1 long terminal repeat (LTR) [44]. Thus, we believe that the presence of Tat in the vector does not detract from the main conclusions of the present work, particularly since Tat has been described to enhance HIV-1 replication and cell killing, rather than protecting cells from viral infection [50–52]. Future studies will examine Tat-defective constructs using different reporter gene cassettes.
A second viral gene, vpu, was also included in the DHIV-CTE vector. To assess the potential role of vpu in vector-mediated gene transduction and inhibition of wild-type virus replication, DHIV-CTE was modified by ablation of the vpu gene, thereby generating DHIV-CTEΔvpu. Vpu was found to be dispensable for vector production and transduction of MDM, as previously reported by An et al. [44]. However, challenge experiments with infectious HIV-1 revealed that DHIV-CTEΔvpu-transduced MDM were very slightly less resistant to wild-type virus infection than DHIV-CTE-transduced cells. This suggests that vpu may contribute to the observed inhibition of wild-type HIV-1 at very limited level by our DLV constructs. Possible reasons might include the fact that Vpu is involved in CD4 receptor degradation [53–56].
Overall, the mechanistic basis for the inhibitory effect of our DLV vectors on the replication of wild-type HIV-1 remains uncertain; possible reasons may include competition for necessary intracellular cofactors and/or preferential replication or packaging of the DLV genomes. Future experiments will be needed to address this. Such studies will also allow for the optimization of DLV-based approaches to the inhibition of HIV-1 replication, which is presently considerably less efficient than other antiviral approaches such as siRNA or dominant negative mutants [12,57].
In conclusion, we have developed an optimized strategy which allows for highly efficient ex vivo transduction of primary human monocyte-derived macrophages with defective HIV-1-based lentivirus vectors pseudotyped with the VSV-G glycoprotein. Using this method, we obtained stable long-term gene expression in cultured human MDM. Furthermore, we have demonstrated that DLV-transduced primary MDM cultures are at least partially refractory to infection by wild-type HIV-1. These findings will form the basis for future in vivo studies, in which we intend to explore the ability of DLV-transduced MDM to cross the BBB and enter the CNS, thereby acting as a novel delivery system for CNS gene therapy.
Acknowledgments
This study was supported by grants from the National Institutes of Health (S11NS043499, G12RR003061, P01MH064570 and R01AI049057) and by the Hawaii Community Foundation.
References
- 1.Akkina RK, Walton RM, Chen ML, et al. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol. 1996;70:2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutton RE, Wu HTM, Rigg R, et al. Human immunodeficiency virus type 1 vectors efficiently transduced human hematopoietic stem cells. J Virol. 1998;72:5781–5788. doi: 10.1128/jvi.72.7.5781-5788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas DL, Case SS, Crooks GM, et al. Critical factors influencing stable transduction of human CD34+ cells with HIV-1-derived lentiviral vectors. Mol Ther. 2000;2:71–80. doi: 10.1006/mthe.2000.0094. [DOI] [PubMed] [Google Scholar]
- 4.Zhang XY, La Russa VF, Bao L, et al. Lentiviral vectors for sustained transgene expression in human bone marrow-derived stromal cells. Mol Ther. 2002;5:555–565. doi: 10.1006/mthe.2002.0585. [DOI] [PubMed] [Google Scholar]
- 5.Deola S, Scaramuzza S, Birolo RS, et al. Mobilized blood CD34+ cells transduced and selected with a clinically applicable protocol reconstitute lymphopoiesis in SCID-Hu mice. Hum Gene Ther. 2004;15:305–311. doi: 10.1089/104303404322886156. [DOI] [PubMed] [Google Scholar]
- 6.Hong Y, Lee K, Yu SS, et al. Factors affecting retrovirus-mediated gene transfer to human CD34+ cells. J Gene Med. 2004;6:724–733. doi: 10.1002/jgm.549. [DOI] [PubMed] [Google Scholar]
- 7.Lu X, Humeau L, Slepushkin V, et al. Safe two-plasmid production for the first clinical lentivirus vector that achieves >99% transduction in primary cells using a one-step protocol. J Gene Med. 2004;6:963–973. doi: 10.1002/jgm.593. [DOI] [PubMed] [Google Scholar]
- 8.Neil S, Martin F, Ikeda Y, et al. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J Virol. 2001;75:5448–5456. doi: 10.1128/JVI.75.12.5448-5456.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mordelet E, Kissa K, Calvo CF, et al. Brain engraftment of autologous macrophages transduced with a lentiviral flap vector: an approach to complement brain dysfunctions. Gene Ther. 2002;9:46–52. doi: 10.1038/sj.gt.3301591. [DOI] [PubMed] [Google Scholar]
- 10.Lee MTM, Coburn GA, McClure MO, et al. Inhibition of human immunodeficiency virus type 1 replication in primary macrophages by using Tat- or CCR5-specific small interfering RNAs expression from a lentivirus vector. J Virol. 2003;77:11964–11972. doi: 10.1128/JVI.77.22.11964-11972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroers R, Kissa K, Calvo CF, et al. Transduction of human PBMC-derived dendritic cells and macrophages by an HIV-1-based lentiviral vector system. Mol Ther. 2000;1:171–179. doi: 10.1006/mthe.2000.0027. [DOI] [PubMed] [Google Scholar]
- 12.Banerjea A, Li ML, Bauer G, et al. Inhibition of HIV-1 by lentiviral vector-transduced siRNA in T lymphocytes differentiated in SCID-hu mice and CD34+ progenitor cell-derived macrophages. Mol Ther. 2003;8:62–71. doi: 10.1016/s1525-0016(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y, Liu C, Zeng L, et al. Efficient gene transfer into human monocytes-derived macrophages using defective lentiviral vectors. Cell Mol Biol. 2003;49:115–1156. [PubMed] [Google Scholar]
- 14.Buchschacher GL, Wong-stall F. Development of lentiviral vectors for gene therapy for human diseases. Blood. 2000;95:2499–2504. [PubMed] [Google Scholar]
- 15.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of tuning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 16.Nabel GJ. Genetic, cellular and immune approaches to disease the past and future. Nat Med. 2004;10:135–141. doi: 10.1038/nm990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gartner S, Markovits P, Markovits DM, et al. The role of mononuclear phagocytes in HTLV-III/LAW infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 18.Gendelman HE, Orenstein JM, Martin MA, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collman R, Hassan NF, Walker R, et al. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1): monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989;170:1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubois-Dalcq M, Altmeyer R, Chiron M, et al. HIV interactions with cells of the nervous system. Curr Opin Neurol. 1995;5:647–755. doi: 10.1016/0959-4388(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhu T. HIV-1 genotypes in peripheral blood monocytes. J Leukoc Biol. 2000;68:338–344. [PubMed] [Google Scholar]
- 22.McArthur JC, Sacktor N, Selnes O. Human immunodeficiency virus-associated dementia. Semin Neurol. 1999;19:129–136. doi: 10.1055/s-2008-1040831. [DOI] [PubMed] [Google Scholar]
- 23.Portegies P, Solod L, Cinque P, et al. Guidelines for the diagnosis and management of neurological complications of HIV infection. Eur J Neurol. 2004;11:297–304. doi: 10.1111/j.1468-1331.2004.00856.x. [DOI] [PubMed] [Google Scholar]
- 24.Dore GJ, McDonald A, Li Y, et al. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 2003;17:1539–1545. doi: 10.1097/00002030-200307040-00015. [DOI] [PubMed] [Google Scholar]
- 25.Neuenburg JK, Brodt HR, Herndier BG, et al. HIV-related neuropathology, 1985 to 1999: rising prevalence of HIV encephalopathy in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31:171–177. doi: 10.1097/00126334-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 26.Schneider SD, Rusconi S, Seger RA, et al. Adenovirus-mediated gene transfer into monocytes-derived macrophage of patients with X-linked chronic granulomatous disease: ex vivo correction of deficient respiratory burst. Gene Ther. 1997;4:524–532. doi: 10.1038/sj.gt.3300432. [DOI] [PubMed] [Google Scholar]
- 27.Heider H, Verca SB, Rusconi S, et al. Comparison of lipid-mediated and adenoviral gene transfer in human monocyte-derived macrophages and COS-7 cells. Biotechniques. 2000;28:260–265. 268–270. doi: 10.2144/00282st02. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Santin AD, Mane M, et al. Transduction and utility of the granulocyte-macrophage colony-stimulating factor gene into monocytes and dendritic cells by adeno-associated virus. J Interferon Cytokine Res. 2000;20:21–30. doi: 10.1089/107999000312702. [DOI] [PubMed] [Google Scholar]
- 29.Paul S, Bizouarne N, Dott K, et al. Redirected cellular cytotoxicity by infection of effector cells with a recombinant vaccinia virus encoding a tumor-specific monoclonal antibody. Cancer Gene Ther. 2000;7:615–623. doi: 10.1038/sj.cgt.7700161. [DOI] [PubMed] [Google Scholar]
- 30.Costantini LC, Bakowska JC, Breakefield XO, et al. Gene therapy in the CNS. Gene Ther. 2000;7:93–109. doi: 10.1038/sj.gt.3301119. [DOI] [PubMed] [Google Scholar]
- 31.Ponnazhagan S, Mahendra G, Curiel DT, et al. Adeno-associated virus type 2-mediated transduction of human monocyte-derived dendritic cells: implications for ex vivo immunotherapy. J Virol. 2001;75:9493–9501. doi: 10.1128/JVI.75.19.9493-9501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klimatcheva E, Planelles V, Day SL, et al. Defective lentiviral vectors are efficiently trafficked by HIV-1 and inhibit its replication. Mol Ther. 2001;3:928–939. doi: 10.1006/mthe.2001.0344. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y, Feuer G, Day SL, et al. Multigene lentiviral vectors based on differential splicing and translational control. Mol Ther. 2001;4:375–382. doi: 10.1006/mthe.2001.0469. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez SH, Sanchez JF, Dimitri CA, et al. Neurotrophins prevent HIV Tat-induced neuronal apoptosis via a nuclear factor-kappaB (NF-kappaB)-dependent mechanism. J Neurochem. 2001;78:874–879. doi: 10.1046/j.1471-4159.2001.00467.x. [DOI] [PubMed] [Google Scholar]
- 35.Maggirwar SB, Tong N, Ramirez S, et al. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamagishi S, Matsumoto T, Yokomaku D, et al. Comparison of inhibitory effects of brain-derived neurotrophic factor and insulin-like growth factor on low potassium-induced apoptosis and activation of p38 MAPK and c-Jun in cultured cerebellar granule neurons. Brain Res Mol Brain Res. 2003;119:184–191. doi: 10.1016/j.molbrainres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Humeau LM, Binder GK, Lu X, et al. Efficient lentiviral vector-mediated control of HIV-1 replication in CD4 lymphocytes from diverse HIV+ infected patients grouped according to CD4 count and viral load. Mol Ther. 2004;9:902–913. doi: 10.1016/j.ymthe.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Azzouz M, Kingsman SM, Mazarakis ND. Lentiviral vectors for treating and modeling human CNS disorders. J Gene Med. 2004;6:951–962. doi: 10.1002/jgm.600. [DOI] [PubMed] [Google Scholar]
- 39.Triques K, Stevenson M. Characterization of restrictions to human immunodeficiency virus type-1 infection of monocytes. J Virol. 2004;78:5523–5527. doi: 10.1128/JVI.78.10.5523-5527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry SW, Hamilton JA, Tjoelker LW, et al. Platelet-activating factor receptor activation. An initiator step in HIV-1 neuropathogenesis. J Biol Chem. 1998;273:17 660–17 664. doi: 10.1074/jbc.273.28.17660. [DOI] [PubMed] [Google Scholar]
- 41.Dropulic B, Hermankova M, Pitha PA. A conditionally replicating HIV-1 vector interferes with wild-type HIV-1 replication and spread. Proc Natl Acad Sci U S A. 1996;93:11 103–11 108. doi: 10.1073/pnas.93.20.11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mautino MR. Lentiviral vectors for gene therapy of HIV-1 infection. Curr Gene Ther. 2002;2:23–43. doi: 10.2174/1566523023348165. [DOI] [PubMed] [Google Scholar]
- 43.Barker E, Planelles V. Vectors derived from human immunodeficiency virus, HIV-1. Front Biosci. 2003;8:491–510. doi: 10.2741/939. [DOI] [PubMed] [Google Scholar]
- 44.An DS, Morizono K, Li QX, et al. An inducible HIV vector which effectively suppresses HIV replication. J Virol. 1999;73:7671–7677. doi: 10.1128/jvi.73.9.7671-7677.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbeau P, Wong-Staal F. Anti-HIV effects of HIV vectors. Virology. 1998;243:268–274. doi: 10.1006/viro.1998.9089. [DOI] [PubMed] [Google Scholar]
- 46.Bukovsky AA, Song JP, Naldini L. Interaction of HIV-derived vectors with wild-type virus in transduced cells. J Virol. 1999;73:7087–7092. doi: 10.1128/jvi.73.8.7087-7092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volsky DJ, Simm M, Shahabuddin M, et al. Interference to human immunodeficiency virus type 1 infection in the absence of downmodulation of the principal virus receptor, CD4. J Virol. 1996;70:3823–3833. doi: 10.1128/jvi.70.6.3823-3833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoglund S, Ohagen A, Goncalves J, et al. Ultrastructure of HIV-1 genomic RNA. Virology. 1997;233:271–279. doi: 10.1006/viro.1997.8585. [DOI] [PubMed] [Google Scholar]
- 49.Ding SF, Noronha J, Joshi S. Co-packaging of sense and antisense RNAs: a novel strategy for blocking HIV-1 replication. Nucleic Acids Res. 1998;26:3270–3278. doi: 10.1093/nar/26.13.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang L, Bosch I, Hofmann W, et al. Tat protein induces HIV-1 coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J Virol. 1998;72:8952–8960. doi: 10.1128/jvi.72.11.8952-8960.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li CJ, Ueda Y, Shi B, et al. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc Natl Acad Sci U S A. 1997;94:8116–8120. doi: 10.1073/pnas.94.15.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westendorp MO, Frank R, Ochsenbauer C, et al. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 53.Maldarelli F, Chen MY, Willey RL, et al. Human immunodeficiency virus type 1 Vpu protein is an oligomeric type I integral membrane protein. J Virol. 1993;67:5056–5061. doi: 10.1128/jvi.67.8.5056-5061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willey RL, Maldarelli F, Martin MA, et al. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Margottin F, Bour SP, Durand H, et al. Novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- 56.Freed EO, Martin MA. HIVs and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; Baltimore: 2001. pp. 1971–2094. [Google Scholar]
- 57.Nishitsuji H, Ikeda T, Miyoshi H, et al. Expression of small hairpin RNA by lentivirus-based vector confers efficient and stable gene-suppression of HIV-1 on human cells including primary non-dividing cells. Microbes Infect. 2004;6:76–85. doi: 10.1016/j.micinf.2003.10.009. [DOI] [PubMed] [Google Scholar]