Abstract
The ovarian surface epithelium (OSE) has a prominent role in ovarian cancer in women, but no studies have been conducted to evaluate its role in normal ovarian function. Data from other species suggest the OSE is needed for ovulation. We have tested whether the OSE is needed for follicle rupture, a necessary step in ovulation, using the nonhuman primate, rhesus macaque. The OSE was removed in two different short-term protocols spanning a single periovulatory interval—one protocol used a cytology brush to remove the OSE only from the follicle apex, and one used mild detergent to remove the entire OSE—and in one long-term protocol spanning 6 wk (two periovulatory intervals) that removed the entire OSE with detergent. Serum levels of estrogen and progesterone (E and P) were monitored, and sectioned ovaries were examined for evidence of successful OSE removal and follicle rupture. In the short-term protocols, removal of the OSE over the follicle apex did not prevent follicle rupture (n = 4 ovaries), but removal of the entire OSE using detergent did in four of six cases. In the long-term protocol, when ovaries were collected after the second periovulatory interval, all the ovaries (n = 5) showed evidence of follicle rupture. In all the protocols, E and P production appeared unaffected. Detergent penetrated up to 40 μm into the ovary. This may have transiently disrupted the stroma and caused follicle rupture failure. We conclude that the primate OSE is not essential for ovulation and perhaps can be removed without lasting consequence.
Keywords: ovary, ovulation, primate, surface epithelium
The ovarian surface epithelium can be removed from primate ovaries without preventing ovulation.
INTRODUCTION
No role has been demonstrated for the ovarian surface epithelium (OSE) in women, in large part because of the difficulty of studying it in vivo. However, data from a number of species—including common laboratory and domestic animals—suggest that the OSE may be required for ovarian function (primarily ovulation and tissue repair) and may protect the ovary from the wounding aspects of ovulation [1–8]. Whether the OSE in women is necessary for ovulation and/or ovarian health has clinical significance; defects in the OSE could underlie some instances of reduced fertility, or the OSE could be a novel target for contraceptive strategies. While it may be that the primate OSE participates in ovulation, there remains the possibility that it does not. In fact, the primate OSE may not be an essential component of the ovary. If so, then elimination of the OSE could provide a strategy to reduce epithelial ovarian cancer risk—the most common ovarian cancer affecting women.
A direct assessment of the role of the OSE in women is not feasible, and nonprimate animal models may not accurately represent the primate OSE due to fundamental differences between primate and nonprimate reproductive physiologies, ovarian biology, and the OSE itself. Notably, epithelial ovarian cancer has been reported in nonhuman primates [9] and hens, but is absent or exceedingly rare in most species. We have selected the nonhuman primate, rhesus monkey, as a surrogate system to investigate whether the OSE is required for follicle rupture, as an indicator of ovulation, and/or normal estrogen and progesterone (E and P) production. The rhesus monkey has reproductive and ovarian physiologies that are highly similar to those of women [10], and its OSE shares gene expression patterns with human OSE in vivo and in vitro [11, 12]. In order to determine whether the OSE is required for these processes, we eliminated the OSE over the follicle apex or from the entire ovary shortly before enacting a controlled ovulation (COv) protocol that precisely times ovulation-triggering events. We also examined the long-term effects of OSE removal on follicle rupture and E and P production by removing the OSE entirely during the early follicular phase and collecting ovaries approximately 6 wk later to allow for one-and-a-half menstrual cycles, i.e., two potential ovulations per ovary. Histological examination of ovarian tissue sections was used to determine whether follicle rupture occurred and what effects OSE removal had on the ovary in general, such as scarring or adhesion formation. Immunhistochemical (IHC) analysis was used to evaluate the effectiveness of OSE removal. These studies provide novel findings on the role of the primate OSE in ovulatory events.
MATERIALS AND METHODS
In Vivo Protocols and Tissue Collection
Rhesus monkeys were cared for and housed by the Division of Animal Resources at the Oregon National Primate Research Center (ONPRC) as described previously [13], and all the experimental protocols were approved by the ONPRC Institutional Animal Care and Use Committee. Adult female rhesus monkeys aged 6–14 yr were monitored for menstrual cyclicity, using visual evidence of menses and serum assays for estradiol and progesterone [12].
The short-term effects of epitheliectomy were examined by removing the OSE during a controlled ovulation (COv) procedure that times ovulation-triggering events while maintaining the dominant follicle selected to ovulate and allowing it to undergo normal periovulatory steroidogenesis [14, 15]. Briefly, the gonadotropin releasing hormone antagonist, acyline (s.c. 75 μg/kg/day; donated by the Contraception and Reproductive Health Branch, Center for Population Research NIH/NICHD, Bethesda, MD), follicle-stimulating hormone and luteinizing hormone (each i.m. 30 IU/day; Ferring Pharmaceuticals, Saint-Prex, Switzerland), and human chorionic gonadtropin (i.m. 1000 IU; Ferring Pharmaceuticals) were injected under this defined protocol to induce a timed ovulation. In the first protocol (protocol 1), the ovary (n = 4) was gently brushed during laparoscopy essentially as described previously [12], except the OSE was removed only at the apex of the preovulatory follicle, and it was removed during the COv. In the second protocol (protocol 2), the ovary (n = 6) was bathed in 1% SDS in sterile saline for 5 min during laparotomy to remove the entire OSE. Gentle abrasion using a cytology brush was used concurrently to facilitate OSE removal. A surgical glove was modified to isolate the ovary and detergent from the peritoneal cavity, and the ovary was rinsed liberally to eliminate residual detergent.
The long-term effects of epitheliectomy were examined by removing the entire OSE with detergent and mild abrasion during the early follicular phase. Hemiovariectomized females, which cycle normally, were used for this procedure to ensure that the remaining, treated ovary would contain the follicle destined to ovulate during each cycle. In this protocol (protocol 3), the ovary was collected approximately 6 wk following the treatment, allowing progression through one complete cycle, an additional follicular phase, and entry into a second luteal phase (when P reached 2–3 ng/ml after an E surge).
Blood samples were collected daily (protocols 1 and 2) or every 2–3 days (protocol 3) until ovary collection, and serum levels of E and P were determined by the Endocrine Technology Core Laboratory at ONPRC as described [15]. In all the protocols, the ovaries were collected during laparoscopy from anesthetized animals under approved conditions [16] with attention given to preserving the ovarian surface and protecting any remaining surface epithelium.
Collected ovaries were fixed for 24 h at 4°C in formaldehyde (38% solution, Fisher Scientific, Pittsburg, PA) diluted 1:1 in PBS-Triton (0.6% Triton X-100, 1.8 mM NaH2PO4, 8.4 mM Na2HPO4, and 175 mM NaCl) and adjusted to pH 9 with NaOH. Tissue was then maintained in 10% sucrose for 24 h, dehydrated in ethanol, equilibrated in citrus-clearing solvent (Richard-Allan Scientific, Kalamazoo, MI), and imbedded in paraffin. Tissue was cut into 10-μm sections in the Imaging and Morphology Core Laboratory at the ONPRC using a Microm HM325 microtome.
Tissue Analysis
Ovarian tissue was processed for hematoxylin and eosin (H&E) staining or IHC probing as described [12]. The following primary antibodies were applied overnight at 4°C, diluted 1:1000: cytokeratin (DAKO Corp., Carpinteria, CA), N-cadherin, E-cadherin, and β-catenin (Transduction Laboratories, Lexington, KY). Phosphatase-conjugated secondary antibodies (Kirkegaard & Perry Laboratories, Gaithersburg, MD) were used, and the signal was detected using a NBT/BCIP (nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indoylphospate, toluidine salt) premixed solution (Kirkegaard & Perry Laboratories). As a negative control for nonspecific antibody labeling, secondary antibodies were applied in the absence of primary antibodies and reacted.
Tissue sections were visualized using an inverted Olympus IX71 microscope with Nomarski differential interference contrast and relief contrast filters (Olympus, Center Valley, PA). Digital images were captured with an Olympus Microfire camera connected to an Apple iMac G5 (Apple, Cupertino, CA) operating PictureFrame 2.0 (Optronics, Goleta, CA).
Serial sections were examined to identify sites of follicle rupture, granulosa cell hypertrophy (indicative of corpus luteum development), the status of the surface epithelium, and general histological evidence of trauma, damage, or other effects of OSE ablation. OSE removal was quantified by determining the linear distance along the ovarian surface in each section analyzed (a minimum of 20 sections per ovary) that was OSE positive versus OSE negative.
Statistical Analysis
Changes in E and P production were analyzed by ANOVA, followed by Student t-test where appropriate. The statistical software used was SigmaStat 2.0 and Microsoft Excel 2008.
RESULTS
Markers for the Primate OSE In Vivo
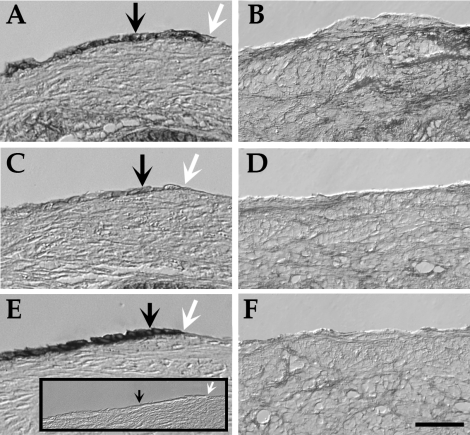
We previously used several markers to detect the OSE in frozen, fixed human and rhesus ovarian tissue [12], and we tested these markers for the current study on paraffin-embedded sections (Fig. 1). β-catenin labeled the OSE effectively but was also detected in other ovarian cell types. N-Cadherin was detected in approximately 50% of OSE cells, while E-cadherin was expressed in most OSE cells in vivo. Keratin was another marker that labeled the OSE but not the underlying stroma (not shown, but see [12]). Together these antigens provided a robust identification of the OSE and readily identified where the OSE had been removed from the ovarian surface.
FIG. 1.
Markers for the OSE in vivo can distinguish between (A, C, and E) unbrushed and (B, D, and F) brushed ovarian surface. Markers shown are (A and B) β-catenin, (C and D) N-cadherin, and (E and F) E-cadherin. Inset is secondary antibody alone. Black arrows indicate OSE, and white arrows show a transition between a brushed and unbrushed surface. Bar = 50 μm.
Short-Term Effects of Epitheliectomy on Estrogen and Progesterone
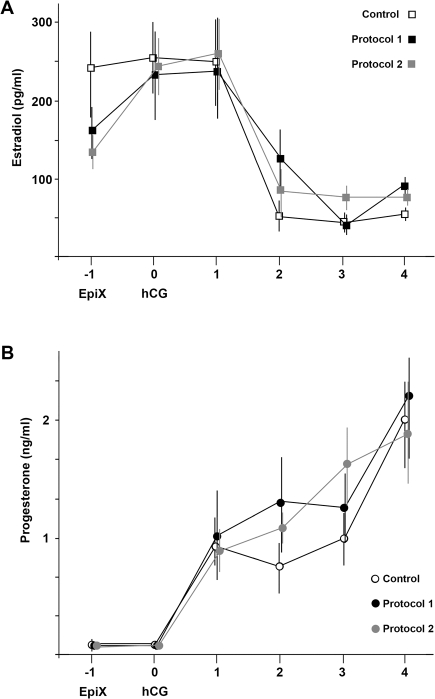
Rhesus monkeys undergoing COv and partial or complete OSE ablation (protocols 1 and 2) had normal patterns of post- hCG (human chorionic gonadotropin) E and P levels in the serum, consistent with an unimpeded ability of the ovary to produce these sex steroids (Fig. 2). Mean values of serum E were 228–258 pg/ml on the day of and the day after hCG, falling to less than 100 pg/ml within 4 days of hCG. Serum P levels were statistically indistinguishable between groups, rising to 1 ng/ml the day after hCG, and rising to approximately 2 ng/ml within 4 days, consistent with normal early luteal development, even after OSE ablation.
FIG. 2.
Serum levels of (A) estradiol and (B) progesterone in rhesus monkeys undergoing controlled ovulation (COv; control), COv plus acute epitheliectomy (EpiX) using a cytology brush (protocol 1), or SDS and mild abrasion (protocol 2). EpiX marks the day of initial surgery and injection of acyline, follicle-stimulating hormone, and luteinizing hormone. Human chorionic gonadotropin (hCG) marks the day a bolus of hCG was administered to trigger ovulation. For each data point, n = 4–6; error bars denote the standard deviation. No statistical differences in estradiol or progesterone levels were detected as a result of EpiX in either protocol.
Short-Term Effects of Partial Epitheliectomy at the Follicle Apex
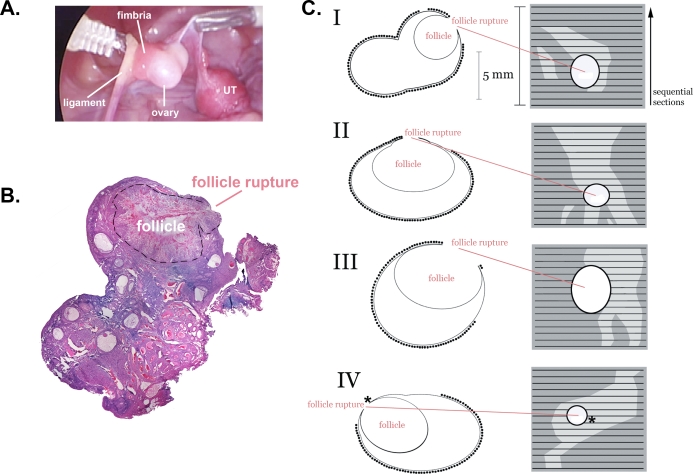
The effectiveness of OSE removal at the follicle apex using a cytology brush (protocol 1) was evaluated by H&E staining and by IHC (Fig. 3). The apex of the preovulatory follicle could be readily identified during laparoscopy (Fig. 3A), and the status of the follicle (ruptured or not) could be determined conclusively after sectioning (Fig. 3B). Using H&E and markers for the OSE, we were able to label serial sections and identify regions where the OSE had been successfully eliminated (Fig. 3C). We found that the OSE on the ovary was typically more than 90% intact, but where brushing occurred at the follicle apex more than 90% of the OSE was removed. After brushing, ruptured follicles were observed in all instances (n = 4). In three out of four instances, rupture occurred at the edge of brushed regions (Fig. 3C). In the remaining instance, the targeted region encompassed the site of rupture; however, detailed examination revealed the presence of small numbers of residual OSE cells at the site of follicle rupture. Thus, follicle rupture occurred either at the edge of brushed regions or in the presence of residual OSE cells within brushed regions.
FIG. 3.
Schematic reconstruction of the extent of EpiX at the apex of the preovulatory follicle on four ovaries (I–IV) using a cytology brush (protocol 1). A) Photograph of ovary I during laparoscopic EpiX, showing the tip of the cytology brush, ovary, ovarian ligament, fimbria, and uterus (UT). B) Photomontage of a H&E-stained section from ovary I, showing the ruptured follicle (outlined with dashed line). C) Left diagrams represent schematics of representative sections from each ovary, containing the site of follicle rupture. The presence of OSE is denoted by (•). Right panels represent serial reconstructions showing the site of follicle rupture, intact OSE (dark gray), and brushed areas (light gray). Asterisk (*) indicates where a few OSE cells were present at the site of follicle rupture but were too few to be shown to scale in this schematic.
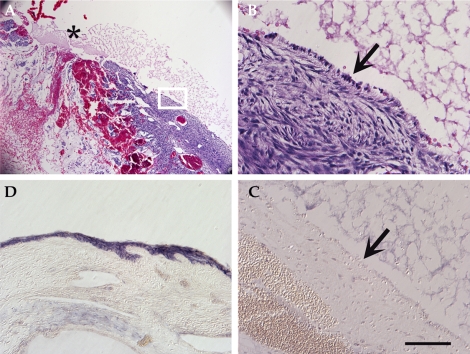
Short-Term Effects of Complete Epitheliectomy Using Mild Detergent
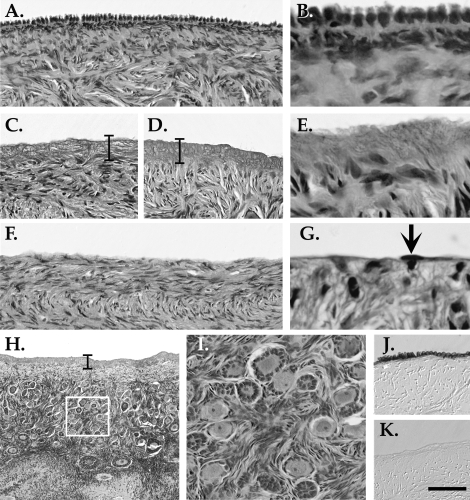
In pilot studies, we observed that the lysing effects of detergent extended slightly into the ovary (≤40 μm beyond the surface), as evidenced by the absence of cell nuclei near the ovarian surface, and therefore the outermost stromal cells were eliminated along with the OSE (Fig. 4). This apparent zone of lysis was not reduced when ovaries were treated for 1 min rather than 5 min (Fig. 4, C vs. D and E). The acellularization of the outer matrix was transient: when ovaries were collected 96 h after SDS-treatment, no evidence of subepithelial lysis was detected (Fig. 4, F and G). Qualitative inspection of treated ovaries gave no indication that the detergent penetrated into cortical layers underlying the outer stroma, and the follicle-rich cortex appeared intact after treatment (Fig. 4, H and I). Immunohistochemistry confirmed the loss of OSE by detergent treatment (Fig. 4, J and K).
FIG. 4.
Cell lysis at the ovarian surface. A) H&E labeling of a tissue section from an untreated ovary, showing normal OSE. B) Higher magnification of A. C and D) H&E labeling after mild abrasion and exposure to 1% SDS for (C) 1 min or (D) 5 min, showing loss of OSE and cell nuclei to a depth of 40 μm into the underlying matrix. Brackets highlight this zone. E) Higher magnification of the slide shown in D. F) H&E labeling of tissue treated with 1% SDS and mild abrasion for 5 min and collected 4 days later, showing an absence of OSE but no cell-free band beneath the ovarian surface. G) Higher magnification of F. Arrow indicates a single cell at the surface. H) Lower magnification of the slide shown in D, showing deeper stromal and cortical layers and early stage follicles. White box indicates the region shown in I, with intact early stage follicles. J and K) E-Cadherin labeling of an (J) untreated ovary and a (K) detergent-treated ovary. Bar = 50 μm, (A, C, D, F, I–K), 10 μm (B, E, G), and 150 μm (H).
Epitheliectomy using detergent (protocol 2) removed all trace of the OSE (>99%). Furthermore, follicle rupture failed to occur in four of six cases, despite elevated P levels that indicated entry into a luteal phase. In the two of six instances where follicle rupture did occur, it appeared normal, aside from a complete absence of OSE cells (Fig. 5). Serum E and P levels on the day of collection for the monkeys with ruptured follicles were 41–83 pg/ml and 0.8–2.5 ng/ml, respectively, while for the monkeys without ruptured follicles they were 47–98 pg/ml and 0.8–2.0 ng/ml, respectively. These serum levels were neither statistically different nor abnormal for the early luteal phase.
FIG. 5.
The OSE and ovulation following acute EpiX using 1% SDS and mild abrasion (protocol 2). A) Low magnification of a follicle rupture site (asterisk) as visualized with H&E stain. B) Higher magnification of the white box in A, showing cells at the ovarian surface (arrow). C) E-Cadherin labeling of a section adjacent to that in B does not identify surface cells as OSE. D) A different area of the slide in C showing a portion of the fimbria, which was not treated with SDS, that retains E-cadherin immunoreactivity. Bar = 400 μm (A) and 80 μm (B–D).
Long-Term Effects of Epitheliectomy Using Mild Detergent
Monkeys with ovaries that underwent SDS-based OSE removal in the early follicular phase displayed normal serum E and P patterns during the menstrual cycle (not shown). Peak estrogen levels that signaled the end of a follicular phase occurred at 12.3 ± 2.2 days after menses, and the next menses was detected at 27.4 ± 1.1 days.
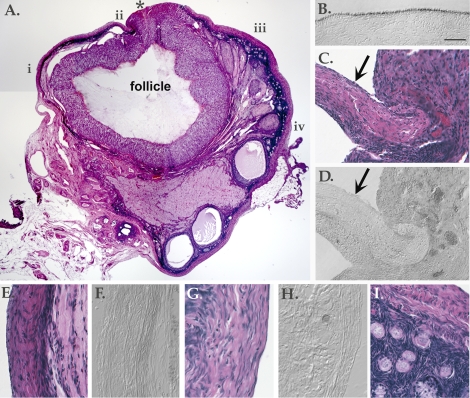
Follicle development and rupture sites appeared normal in ovaries from all animals (n = 5), aside from the absence of OSE (Fig. 6). There was no apparent replacement of the OSE. When cells were observed on the ovarian surface, they did not express markers for the OSE. Even in the few instances when clusters of cells gave the appearance of a monolayered, squamous-to-cuboidal epithelium, IHC showed them to be negative for the OSE markers E-cadherin (Fig. 6, D, F, and H), N-cadherin, β-catenin, and keratin (not shown). Although the identity of surface cells was not confirmed, aside from their lack of normal OSE features, they were more prevalent near the site of follicle rupture and may have originated from the ruptured follicle. No persistent or newly developed effects of detergent were seen in deeper stromal or cortical layers containing primordial and secondary follicles (Fig. 6I), based upon a qualitative inspection. No evidence of adhesions or other tissue damage was seen.
FIG. 6.
Long-term effects of detergent treatment. A) Photomontage of an H&E-labeled section from an ovary treated with 1% SDS for 5 min and collected 6 wk later. Asterisk (*) marks the site of follicle rupture. Areas shown at higher magnification in other panels of this figure are denoted by i–iv. B) E-Cadherin labeling of a section from an untreated ovary. This slide was processed in parallel with other E-cadherin-labeled slides shown in this figure. C) H&E-labeled slide showing area ii. Arrow indicates a group of cells at the surface. D) E-Cadherin labeling showing area ii. Arrow indicates a group of unlabelled cells at the surface. E) H&E-labeled slide showing area i. F) E-Cadherin labeling showing area i. G) H&E-labeled slide showing area iv. H) E-Cadherin labeling showing area iv. I) H&E-labeled slide showing area iii, beneath the surface to display underlying stromal and cortical layers with early stage follicles. Bar = 1 mm (A) and 50 μm (B–I).
DISCUSSION
A direct evaluation of the effects of OSE removal on follicle rupture indicates that the OSE is not essential for this process in rhesus monkeys. This is in contrast to considerable data from nonprimates demonstrating that the OSE may facilitate, or is necessary for, follicle rupture [1–8, 17, 18]. Our findings do not discount that the primate OSE may contribute in some manner to follicle rupture or other processes, such as luteal regression, steroid metabolism, anti-inflammatory activity, and prevention of adhesions. Longer term studies may reveal significant consequences of epitheliectomy.
We found that removal of the OSE at the follicle apex 36–48 h prior to the anticipated time of ovulation using a cytology brush (protocol 1) eliminated most (>90%) of the OSE in targeted regions. It is possible that brushing was slightly more effective than observed because a small amount of repopulation of the ovarian surface by residual OSE cells may have occurred in the 4 days following brushing prior to ovary collection [12]. Despite the effectiveness of brushing, follicle rupture occurred in all cases. In contrast, we found that complete epitheliectomy using a combination of 1% SDS and brushing (protocol 2) in the same time period prevented follicle rupture in four of six instances. However, when ovaries were epitheliectomized via detergent in the early follicular phase (protocol 3; long-term effects), we observed normal patterns of E and P cyclicity throughout the 6-wk protocol and ruptured follicles when ovaries were collected during the second luteal phase.
The disparate effects of total OSE removal in the short-term versus long-term protocols suggest that detergent affects the ovary transiently and that follicle rupture occurs independently of the OSE because the OSE is not restored concurrently with the return of follicle rupture. This raises questions about the nature of follicle rupture and its susceptibility to mild detergent treatment. We observed an initial zone ≤40 μm beneath the ovarian surface that appeared free of cell nuclei as a result of detergent treatment. Since the dominant follicle does not approach within 40 μm of the ovarian surface until the time of ovulation, it is unlikely that detergent acted on the dominant follicle directly. This is supported by the persistent production of E and P by the follicle even after the treatment. It is also unlikely that follicle rupture was prevented by direct action on the collagen matrix surrounding the ovary in such a way that it would resist the proteolytic degradation required for follicle rupture; on the contrary, mild SDS treatment has been shown to enhance the susceptibility of collagen to proteolysis, presumably by unmasking digestion motifs [19, 20].
It seems likely that the transient prevention of follicle rupture may result from the loss of stromal factors residing within 40 μm of the ovarian surface, at the site of pending ovulation. Collagenolytic activity that may be important for ovulation has been detected in the perifollicular stroma and tunica albuginea in women [21] in addition to follicle-derived matrix proteases associated with ovulation in primates [1, 22–24]. The transient effects of detergent are consistent with this. Cells within 40 μm of the ovarian surface would be lysed and the proteins inactivated by the detergent. However, once the detergent is removed, stromal cells repopulate this region within days and newly produced factors would be unaffected by prior detergent treatment.
Even though SDS is biodegradable and safe to use in cosmetics and oral hygiene, and our protocol was mild—clinical acellularization of collagen structures for transplant exposes tissue to more highly concentrated SDS for days or weeks [25, 26]—it indiscriminately denatures proteins and solubilizes cell membranes. Under more stringent conditions, it could be expected to have a longer lasting impact on the ovary due, for example, to increased penetrance that would expose early stage follicles to irreversible lysis. The following evidence suggests that the only long-lasting effect of SDS treatment under current conditions is the elimination of the OSE: 1) the ovaries appeared grossly normal, with no adhesions around the treated ovaries; 2) no evidence of tissue damage was seen at the ovarian surface, in the underlying stroma, or in deeper cortical regions; and 3) cyclic ovarian function was seemingly unaffected as E and P patterns were normal, and in the long-term protocol follicle rupture occurred in a timely manner.
The concept that the OSE may be completely and permanently ablated without sacrificing ovarian function offers a compelling strategy for ovarian cancer prevention. Currently the most effective method to reduce ovarian cancer risk is surgical removal of both ovaries. For women at risk for ovarian cancer, especially those with a family history of breast or ovarian cancer, the protective benefits of surgery may outweigh the surgical risks. In these women, voluntary risk-reducing salpingo-oophorectomy is highly effective [27], and women electing to undergo the procedure, near or after menopause, generally express no regret [28]. However, risk-reduction surgery decisions in premenopausal at-risk women can be complicated by a basic dilemma: whether to delay surgery in favor of maintaining child-bearing potential and continued E and P production, versus protecting against a life-threatening disease [29]. A clinical option to undergo surgical risk reduction by epitheliectomy could not only relieve psychological distress, but also prove cost-effective and extend the lives of women at risk for ovarian cancer.
In order for epitheliectomy to be a clinical option for cancer prevention, further study is needed. Data must demonstrate that no long-term deleterious effects occur; that the OSE can be permanently ablated and/or cells that appear in place of the epithelium do not adopt high risk OSE features; and that epitheliectomy does not cause infertility. This study did not examine whether follicle rupture is accompanied by ovulation or fertility, nor did it systematically investigate the potential effects of detergent on the pool of primordial follicles that reside in the ovarian cortex, near the surface. The last effect may be difficult to investigate directly because it could conceivably take years to manifest; however, it is of critical importance in considering the therapeutic consequences of eliminating the OSE. The nonhuman primate may be a valuable model for such long-term studies, especially in light of this report demonstrating that results from nonprimate studies have not effectively predicted the results of epitheliectomy in the primate.
Footnotes
Supported in part by NIH HD-050356, P51RR000163.
REFERENCES
- Espey LL.Ovarian proteolytic enzymes and ovulation. Biol Reprod 1974; 10: 216–235. [DOI] [PubMed] [Google Scholar]
- Gillett WR, James C, Jetha N, McComb PF.Removal of the ovarian surface epithelium from the rabbit ovary—a cause of adhesions following a standard injury. Hum Reprod 1994; 9: 497–500. [DOI] [PubMed] [Google Scholar]
- Colgin DC, Murdoch WJ.Evidence for a role of the ovarian surface epithelium in the ovulatory mechanism of the sheep: secretion of urokinase-type plasminogen activator. Anim Reprod Sci 1997; 47: 197–204. [DOI] [PubMed] [Google Scholar]
- Gubbay O, Guo W, Rae MT, Niven D, Howie AF, McNeilly AS, Xu L, Hillier SG.Anti-inflammatory and proliferative responses in human and ovine ovarian surface epithelial cells. Reproduction 2004; 128: 607–614. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ.Perturbation of sheep ovarian surface epithelial cells by ovulation: evidence for roles of progesterone and poly(ADP-ribose) polymerase in the restoration of DNA integrity. J Endocrinol 1998; 156: 503–508. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Martinchick JF.Oxidative damage to DNA of ovarian surface epithelial cells affected by ovulation: carcinogenic implication and chemoprevention. Exp Biol Med (Maywood) 2004; 229: 546–552. [DOI] [PubMed] [Google Scholar]
- Osterholzer HO, Johnson JH, Nicosia SV.An autoradiographic study of rabbit ovarian surface epithelium before and after ovulation. Biol Reprod 1985; 33: 729–738. [DOI] [PubMed] [Google Scholar]
- Tan OL, Fleming JS.Proliferating cell nuclear antigen immunoreactivity in the ovarian surface epithelium of mice of varying ages and total lifetime ovulation number following ovulation. Biol Reprod 2004; 71: 1501–1507. [DOI] [PubMed] [Google Scholar]
- Moore CM, Hubbard GB, Leland MM, Dunn BG, Best RG.Spontaneous ovarian tumors in twelve baboons: a review of ovarian neoplasms in non-human primates. J Med Primatol 2003; 32: 48–56. [DOI] [PubMed] [Google Scholar]
- Wu JM, Zelinski MB, Ingram DK, Ottinger MA.Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med (Maywood) 2005; 230: 818–828. [DOI] [PubMed] [Google Scholar]
- Wright JW, Toth-Fejel S, Stouffer RL, Rodland KD.Proliferation of rhesus ovarian surface epithelial cells in culture: lack of mitogenic response to steroid or gonadotropic hormones. Endocrinology 2002; 143: 2198–2207. [DOI] [PubMed] [Google Scholar]
- Wright JW, Pejovic T, Fanton J, Stouffer RL.Induction of proliferation in the primate ovarian surface epithelium in vivo. Hum Reprod 2008; 23: 129–138. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL.In vitro fertilization-embryo transfer in nonhuman primates: the technique and its applications. Mol Reprod Dev 1990; 27: 261–280. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Hess DL, Stouffer RL.Dynamics of periovulatory steroidogenesis in the rhesus monkey follicle after ovarian stimulation. Hum Reprod 1999; 14: 642–649. [DOI] [PubMed] [Google Scholar]
- Young KA, Chaffin CL, Molskness TA, Stouffer RL.Controlled ovulation of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod 2003; 18: 2257–2263. [DOI] [PubMed] [Google Scholar]
- Xu F, Hazzard TM, Evans A, Charnock-Jones S, Smith S, Stouffer RL.Intraovarian actions of anti-angiogenic agents disrupt periovulatory events during the menstrual cycle in monkeys. Contraception 2005; 71: 239–248. [DOI] [PubMed] [Google Scholar]
- Bjersing L, Cajander S.Ovulation and the role of the ovarian surface epithelium. Experientia 1975; 31: 605–608. [DOI] [PubMed] [Google Scholar]
- Mori T, Uchida TA.Ultrastructural observations of fertilization in the Japanese long-fingered bat, Miniopterus schreibersii fuliginosus. J Reprod Fertil 1981; 63: 231–235. [DOI] [PubMed] [Google Scholar]
- Bleeg HS.Non-specific cleavage of collagen by proteinases in the presence of sodium dodecyl sulfate. Scand J Dent Res 1990; 98: 235–241. [DOI] [PubMed] [Google Scholar]
- Nandi PK, Grant ME, Robinson DR.Destabilization of collagen structure by amides and detergents in solution. Int J Pept Protein Res 1985; 25: 206–212. [DOI] [PubMed] [Google Scholar]
- Lind AK, Dahm-Kahler P, Weijdegard B, Sundfeldt K, Brannstrom M.Gelatinases and their tissue inhibitors during human ovulation: increased expression of tissue inhibitor of matrix metalloproteinase-1. Mol Hum Reprod 2006; 12: 725–736. [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Yoshioka S, Sharma SC, Lydon JP, O'Malley BW, Espey LL, Richards JS.Ovulation: a multi-gene, multi-step process. Steroids 2000; 65: 559–570. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL.Luteinizing hormone acts directly at granulosa cells to stimulate periovulatory processes: modulation of luteinizing hormone effects by prostaglandins. Endocrine 2003; 22: 249–256. [DOI] [PubMed] [Google Scholar]
- Young KA, Hennebold JD, Stouffer RL.Dynamic expression of mRNAs and proteins for matrix metalloproteinases and their tissue inhibitors in the primate corpus luteum during the menstrual cycle. Mol Hum Reprod 2002; 8: 833–840. [DOI] [PubMed] [Google Scholar]
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA.Clinical transplantation of a tissue-engineered airway. Lancet 2008; 372: 2023–2030. [DOI] [PubMed] [Google Scholar]
- Sandmann GH, Eichhorn S, Vogt S, Adamczyk C, Aryee S, Hober M, Milz S, Imhoff AB, Tischer T.Generation and characterization of a human acellular meniscus scaffold for tissue engineering. J Biomed Mater Res 2009; 91: 567–574. [DOI] [PubMed] [Google Scholar]
- Rebbeck TR, Kauff ND, Domchek SM.Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst 2009; 101: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher EM, Babb S, Whelan A, Mutch DG, Rader JS.Prophylactic oophorectomy and ovarian cancer surveillance. Patient perceptions and satisfaction. J Reprod Med 2001; 46: 87–94. [PubMed] [Google Scholar]
- Ray JA, Loescher LJ, Brewer M.Risk-reduction surgery decisions in high-risk women seen for genetic counseling. J Genet Couns 2005; 14: 473–484. [DOI] [PubMed] [Google Scholar]