Abstract
Background and purpose:
Phosphodiesterase (PDE) inhibitors are useful to treat hypoxia-related diseases and are used in experiments studying the effects of oxygen on 3′-5′-cyclic adenosine monophosphate (cAMP) production. We studied the effects of acute hypoxia on cAMP accumulation induced by PDE inhibitors in oxygen-specific chemosensors, the carotid bodies (CBs) and in non-chemosensitive CB-related structures: carotid arteries (CAs) and superior cervical ganglia (SCG).
Experimental approach:
Concentration–response curves for the effects of a non-specific PDE inhibitor [isobutylmethylxanthine (IBMX) ], PDE4 selective inhibitors (rolipram, Ro 20-1724) and a PDE2 selective inhibitor (erythro-9-(2-hydroxy-3-nonyl)adenine) on cAMP levels were obtained in normoxic (20% O2/5% CO2) or hypoxic (5% O2/5% CO2) conditions.
Key results:
Responses to the PDE inhibitors were compatible with the presence of PDE4 in rat CBs, CAs and SCG but in the absence of PDE2 in CAs and CBs. Acute hypoxia enhanced the effects of IBMX and PDE4 inhibitors on cAMP accumulation in CAs and CBs. In SCG, acute hypoxia reduced cAMP accumulation induced by all the four PDE inhibitors tested. Differences between the effects of Ro 20-1724 and rolipram on cAMP were found in CAs and CBs during hypoxia.
Conclusions and implications:
The effects of PDE4 inhibitors could be potentiated or inhibited by acute hypoxia depending on the PDE isoforms of the tissue. The similarities between the characterization of PDE4 inhibitors at the CBs and CAs, under normoxia and hypoxia, did not support a specific role for cAMP in the oxygen-sensing machinery at the CB and suggested that no direct CB-mediated, hyperventilatory, adverse effects would be expected with administration of PDE4 inhibitors.
Keywords: cAMP, carotid body, carotid artery, hypoxia, PDE inhibitors, superior cervical ganglia, rolipram, IBMX, Ro 20-1724, EHNA
Introduction
The carotid body (CB) is a small paired organ located in the bifurcation of the common carotid artery (CA) and is sensitive to changes in blood PO2, PCO2 and pH. Morphologically, CB includes specific chemoreceptor cells (type I or glomus cells), sustentacular cell (type II) vessels that originate from the common CA bifurcation, sensory nerve endings of the carotid sinus nerve and efferent nerve endings from the superior cervical ganglia (SCG) and glossopharyngeal nerve (McDonald and Mitchell, 1975). Arteries and efferent nerve endings at the CB can be considered non-chemosensitive structures in comparison with type I and II cells and the sensory endings of the carotid sinus nerve.
Hypoxia is the primary stimulus that activates CB chemosensors triggering respiratory reflexes in order to adjust ventilation to the metabolic needs. Although the chemosensory mechanism of the CB is poorly understood, several studies suggested that 3′-5′-cyclic adenosine monophosphate (cAMP) plays a modulatory role in the oxygen-sensing machinery in this organ (Delpiano and Acker, 1991; Gonzalez et al., 1994; Chen et al., 1997). cAMP is the second messenger common to metabotropic receptors coupled to adenylyl cyclase (AC, E.C. 4.6.1.1). In the CB, levels of cAMP are regulated by the stimulatory effects of adenosine mediated by A2A and A2B receptors (Monteiro et al., 1996; Chen et al., 1997; Conde et al., 2006), by β-adrenoceptor activity (Mir et al., 1984) and by dopamine D2 and D1 receptors (Mir et al., 1984; Almaraz et al., 1991; Batuca et al., 2003; receptor nomenclature follows Alexander et al., 2008).
Hydrolysis of cAMP is mediated by cyclic nucleotide phosphodiesterases (PDE, E.C. 3.1.4.17). So far, 11 kinetically distinct PDE isoforms have been identified. These isoforms differ in their affinity for substrates [cAMP and/or 3′-5′-cyclic guanosine monophosphate (cGMP)], their response to modulators and their pharmacological inhibitors (Bender and Beavo, 2006).
PDE inhibitors are useful in the treatment of several pathological conditions related to hypoxia such as pulmonary hypertension, chronic obstructive pulmonary disease and asthma (see Jeon et al., 2005) and are also common tools used to indirectly study AC activity in response to different oxygen concentrations.
Frequently, studies measuring cAMP in the CB under acute hypoxic and normoxic conditions have used the non-isoform-selective PDE inhibitor, isobutylmethylxanthine (IBMX, 500 µM) (Mir et al., 1984; Pérez-Garcia et al., 1990; 1991; Delpiano and Acker, 1991; Batuca et al., 2003). However, PDE activities of the CB have never been characterized and the interpretation of the effects of IBMX could also be confused by changes in PDE activity caused by hypoxia itself. For instance, acute hypoxia (20 min) increased the activity of a Ca2+-dependent PDE in CB (Hanbauer and Lovenberg, 1977), and chronic hypoxia (7 or 12 days) regulates PDE4 activity in pulmonary arteries (Millen et al., 2006) as well as the activity of cAMP-PDE in rat blood (Spoto et al., 1998).
In this study, a pharmacological characterization of the PDE activity present in whole CB, CA and SCG was performed in normoxic and acute hypoxic conditions. CAs and SCG were used as non-chemosensitive structures related to the CBs. This methodological approach has been used previously (Conde and Monteiro, 2004), due to the small size of rat CBs. PDE in these tissues has never been characterized and the effects of oxygen concentrations on their PDE activity are unknown. If cAMP accumulation is relevant to the specificity of oxygen-sensing machinery in chemosensitive tissues, an effect of hypoxia on cAMP catalytic enzymes could be expected.
Manipulation of cyclic nucleotides with PDE inhibitors has been used to induce vasodilation in clinical studies, but the functional effects of cAMP-PDE inhibitors during different oxygen exposures are not known. The long forms of the PDE4 sub-types can be phosphorylated by protein kinase A, causing a conformational change and a 60% to 250% increase in activity of the catalytic domain that leads to an increased affinity for rolipram (Bender and Beavo, 2006). We hypothesized that acute (30 min) changes in oxygen concentrations could affect the degree of PDE phosphorylation and, consequently, the potency and efficacy of PDE inhibitors.
The results of this work will contribute towards interpreting data obtained when PDE inhibitors are used as tools to manipulate cAMP in these tissues, to characterize PDE in oxygen-chemosensitive and non-chemosensitive tissues and to study the potential tissue-specific modulation of PDE inhibitors in hypoxia-related disease conditions. A preliminary report of some of these findings has been published in the proceedings of a conference (Nunes et al., 2009).
Methods
Surgical procedures
All animal care and experimental procedures were carried out in accordance with the European Union directives for the use of experimental animals (Portuguese laws no. 1005/92 and 1131/97). We used CBs, SCG and CAs, isolated from adult (approximately 3 months old) male and female Wistar rats (n= 188). Rats were obtained from the Faculty of Medical Sciences animal facilities, kept at a constant temperature (21°C) with a regular light (08–20 h) and dark (20–08 h) cycle, with food and water ad libitum. Surgical procedures for isolation of the tissues in situ have been described previously (Batuca et al., 2003; Conde and Monteiro, 2004; 2006;). In brief, rats were anaesthetized with sodium pentobarbital (60 mg·kg−1 i.p.), then tracheostomized to allow spontaneous breathing during the surgical procedure (approximately 30 min). After the CBs, SCG and CAs had been removed, the animals were killed by an intracardiac injection of a lethal dose of pentobarbital. The tissues removed with intact endothelium were weighed (the mean weight of the rat CBs, CAs and SCG were 148 µg, 1.579 mg and 1.360 mg respectively) and further prepared as follows.
Effects on cAMP content of oxygen concentrations during the recovery pre-incubation period
The rat CBs, SCG and CAs were removed in vivo and pre-incubated to allow recovery from the surgical procedures in a 37°C shaker bath for 15 min in a medium containing (in mM): NaCl 116, NaHCO3 24, KCl 5, CaCl2 2, MgCl2 1.1, HEPES 10, glucose 5.5 and adjusted to pH 7.40 (Pérez-Garcia et al., 1990). This medium composition was used in all experiments throughout the work. Two groups of experiments were performed in order to study the effect of oxygen concentrations: hyperoxia (95% O2/5% CO2, PO2≈ 677 mmHg) and normoxia (20%O2/5%CO2, PO2≈ 142 mmHg) during the pre-incubation period. After the pre-incubation period, all the CBs, SCG and CAs were incubated in a fresh incubation medium containing 500 µM of IBMX, a non-selective PDE inhibitor, equilibrated in normoxia or hypoxia (5% O2/5% CO2, PO2= 33 mmHg) for 30 min. This incubation period was chosen because we have previously shown that cAMP concentrations obtained during incubations of 30 min were significantly higher than those obtained during 10 min (Batuca et al., 2003) and maintained normal values of cellular viability (Conde and Monteiro, 2004).
Effects of hypoxia on cAMP content in CBs, SCG and CAs in the absence of PDE inhibitors
CBs, SCG and CAs with intact endothelium were removed in vivo and pre-incubated in a 37°C shaker bath in incubation medium (previously described) adjusted to pH 7.40 and equilibrated with 20% O2/5% CO2. After 15 min, the tissues were incubated for 30 min in a fresh medium at 37°C, pH 7.40, in the absence of any inhibitor. The tissues were divided into two experimental groups: one equilibrated in normoxia and the other equilibrated in hypoxia.
Effect of PDE inhibitors on cAMP content in CBs, SCG and CAs
Tissues (CBs, SCG and CAs) previously pre-incubated with 20% O2/5% CO2 for 15 min were incubated in a fresh medium in the presence of PDE inhibitors. The PDE inhibitors used were IBMX (non-selective PDE inhibitor, 0.3–500 µM), Ro 20-1724 (PDE4 selective inhibitor, 0.1–100 µM), rolipram (PDE4 selective inhibitor, 0.1–100 µM) and erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) (PDE2 selective inhibitor, 0.1–100 µM). Only one PDE inhibitor at one concentration was tested in each experiment. The incubation medium containing each PDE inhibitor was equilibrated with 20% O2/5% CO2 or 5% O2/5% CO2 in a shaker bath at 37°C for 30 min, which corresponds to the period necessary to detect changes in the kinetic properties of PDEs (Hanbauer and Lovenberg, 1977).
Cyclic nucleotide extraction and quantification
After incubation, CBs, SCG and CAs were immersed in cold 6% (w/V) trichloroacetic acid (600 µL) for 10 min, weighed on an electrobalance (mc 215 Sartorius, Madrid, Spain), homogenized using a Potter glass homogenizer at 2–8°C and centrifuged at 12 000 g for 10 min at 4°C. The supernatants were washed four times with 3 mL of water saturated with diethyl ether solution (50:50), collected for lyophilization (Christ Alpha 1-2 B. Brawn, Biotech International, Melsungen, Germany) and stored at −20°C until cAMP quantification by enzyme immunoassay, using an EIA commercial kit (RPN 2255 GE Healthcare Bio-Sciences AB, Uppsala, Sweden).
Data analysis and statistical procedures
cAMP content present in CBs, SCG and CAs was expressed in picomoles per milligram of tissue (pmol/mg tissue) instead of mg protein due to the small size of the CB (ca 150 µg, see above). Data were evaluated using Graph Pad Prism (GraphPad Software Inc., version 4, San Diego, CA, USA) and expressed as means ± SEM. Statistical differences between two pre-incubation conditions in the CBs, between normoxic and hypoxic basal cAMP levels in the three different tissues, between cAMP levels from normoxic and hypoxic CAs incubated with 1 µM and 30 µM of IBMX and between normoxic and hypoxic cAMP levels in SCG incubated with 100 µM of EHNA were assessed by the Mann–Whitney non-parametric test. Comparison between the hypoxic effects on different PDE inhibitors in CBs was performed by two-way analysis of variance with Bonferroni's post-test. Concentration–response curves for the effects of inhibitors were analysed using sigmoidal curve-fitting analysis, extrapolating the Emax[mean maximal effect (%) observed in cAMP levels] and the EC50 value (concentration of inhibitor required to elicit 50% of maximum effect observed) for each inhibitor. The comparison between concentration–response curves for the effects of the PDE inhibitors was assessed using Graph Prism to compare the curve-fitted points (log EC50 and Hill slope). The null hypothesis (one curve for all data sets) was rejected on the basis of the F-test. In all cases, P-values of 0.05 or less were considered as significant differences. Also, n corresponds to the number of different experiments performed in each group.
Materials
Sodium pentobarbital, IBMX, 4-[3-(cyclopentyloxy)-4-methoxy-phenyl]-2-pyrrolidinone (rolipram), 4-[(3-butoxy-4-methoxyphenyl)-methyl]-2-imidazolidinone (Ro 20-1724) and EHNA were obtained from Sigma (St. Louis, MO, USA). Rolipram and Ro 20-1724 were dissolved in dimethyl sulphoxide in 1 and 5 mM stock solutions and all subsequent dilutions were made in incubation medium.
Results
Effects of oxygen concentrations during the recovery pre-incubation period on cAMP content
In previous studies where cAMP was quantified, the CBs were allowed to recover from the ischaemia, to which the preparations were submitted during dissection, with a pre-incubation period of 15 min in a medium equilibrated with 100% O2/5% CO2 or 95% O2/5% CO2 (Pérez-Garcia et al., 1990; Almaraz et al., 1991; Monteiro et al., 1996; Chen et al., 1997; Batuca et al., 2003). As hyperoxia is not a physiological condition and prolonged exposure to normobaric hyperoxia attenuates CB chemosensory response to hypoxia (Mokashi and Lahiri, 1991; Torbati et al., 1993; Mokashi et al., 1994), an initial group of experiments was carried out to define the oxygen concentrations to be used during the recovery from ischaemia, in this study. This rationale was applied to the three preparations used in the present work and the results are shown in Figure 1. In these experiments, the non-specific PDE inhibitor IBMX in a high concentration (500 µM) was added to the incubation medium, keeping all the other experimental conditions (recovery and incubation time as well as medium composition) constant throughout the different group of experiments (see Methods section). In light of the absence of statistically significant differences (P > 0.05, Mann–Whitney test) between the two pre-incubation conditions for CBs, CAs and SCG, we decided to perform all the experiments pre-incubating these tissues with 20% O2/5% CO2, which is comparable to an atmospheric oxygen concentration.
Figure 1.
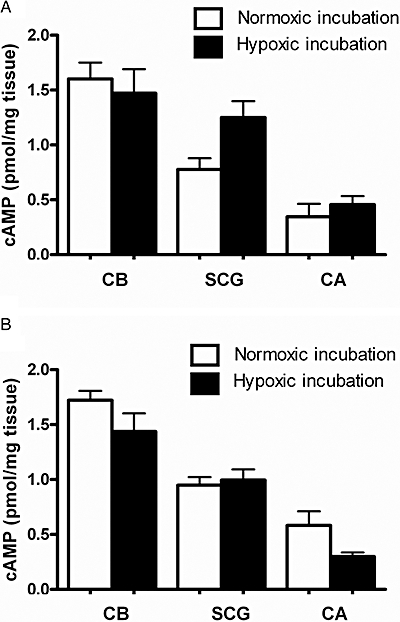
Effect of different pre-incubation conditions on cAMP levels of carotid body (CB, n= 6–9), superior cervical ganglia (SCG, n= 2–5) and carotid artery (CA, n= 4–5) in response to hypoxia (5% O2/5% CO2) and in the presence of isobutylmethylxanthine (500 µM). Tissues were pre-incubated in (A) hyperoxia (95% O2/5% CO2) or (B) normoxia (20% O2/5% CO2). P > 0.05.
Effects of hypoxia on cAMP content in CBs, SCG and CAs in the absence of PDE inhibitors
In the absence of PDE inhibitors, the basal cAMP content observed in CBs, SCG and CAs incubated with 20% O2/5% CO2 is shown in Figure 2. It is apparent here that, in normoxia, cAMP accumulation was higher in CBs than in other tissues (CBs > SCG > CAs) and that hypoxia did not significantly modify cAMP concentrations in either CBs or SCG, but decreased cAMP levels in CAs (P < 0.001). As expected, in these group of experiments, cAMP content in both normoxic and hypoxic conditions was clearly lower, about half that obtained in the presence of 500 µM of IBMX (Figure 1).
Figure 2.
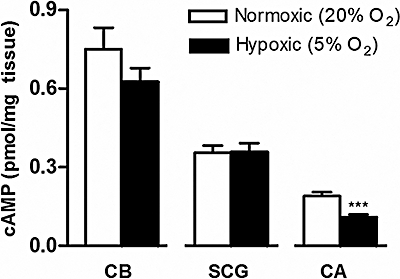
Effects of hypoxia on cAMP levels (expressed as pmol/mg tissue) in rat carotid bodies (CBs, n= 15), superior cervical ganglia (SCG, n= 11) and carotid arteries (CAs, n= 11–12) in the absence of PDE inhibitors. Data represent means ± SEM ***P < 0.001 Mann–Whitney non-parametric test, corresponding to the differences between normoxia and hypoxia.
Effect of PDE inhibitors on cAMP content in CBs
The effects of PDE inhibitors on CBs were assayed indirectly quantifying cAMP content. The concentrations used for each inhibitor were selected according to the published IC50 values: rolipram = 1 µM; Ro 20-1724 = 2 µM; IBMX = 2–50 µM (Bender and Beavo, 2006) and EHNA = 2 µM (Podzuweit et al., 1995). EHNA (0.1–100 µM) did not modify cAMP concentrations in CBs incubated in either normoxia or hypoxia. However, IBMX (0.3–500 µM), rolipram (0.1–100 µM) and Ro 20-1724 (0.1–100 µM) increased cAMP content in CBs in a concentration-dependent manner in normoxia (Figure 3) and hypoxia shifted their concentration–response curves to the left. This shift was statistically significant (P < 0.001) for IBMX and rolipram but not for Ro-20-1724 (Figure 3). This effect of hypoxia was apparent only for concentrations <100 µM for the specific PDE4 inhibitors and higher than 50 µM for the non-selective PDE inhibitor IBMX. The EC50 and Emax values calculated for concentrations between 0.1 and 100 µM for Ro 20-1724 and rolipram and between 0.1 and 500 µM for IBMX are presented in Table 1. The Emax values found in normoxia for concentrations between 0.1 and 100 µM were similar for rolipram, Ro 20-1724 and IBMX (56 ± 11%, 35 ± 8% and 37 ± 13%, respectively, Figure 3). The effect of hypoxia on cAMP accumulation caused by PDE inhibition was more pronounced in the case of rolipram (Figure 3), although no apparent changes in drug potency (EC50) were observed (Table 1). The EC50 values obtained for Ro 20-1724 and rolipram in the CBs were <10 µM (Table 1).
Figure 3.
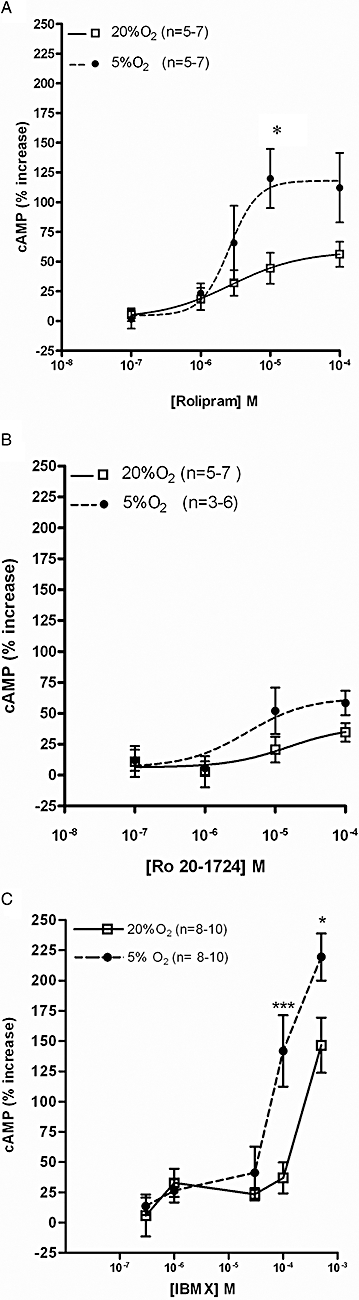
Effects of PDE4 inhibitors on cAMP levels in rat carotid bodies (CBs). Concentration–response curves of (A) rolipram, (B) Ro 20-1724 and (C) isobutylmethylxanthine (IBMX) for effects on cAMP levels induced during normoxia (20%O2) [0% effect − 0.75 ± 0.08 pmol cAMP/mg (n= 15) ] and hypoxia (5%O2) (0% effect − 0.63 ± 0.05 pmol cAMP/mg (n= 15). *P < 0.05 and ***P < 0.001 two-way analysis of variance with Bonferroni's post hoc test. Data values represent means ± SEM.
Table 1.
Comparison between the efficacies and potencies of phosphodiesterase inhibitors (IBMX, rolipram and Ro 20-1724) in normoxia (20% O2) and hypoxia (5% O2)
|
Rolipram (0.1–100 µM) |
Ro 20-1724 (0.1–100 µM) |
IBMX (0.3–500 µM) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
20% O2 |
5% O2 |
20% O2 |
5% O2 |
20% O2 |
5% O2 |
|||||||
| EC50 (µM) | Emax (%) | EC50 (µM) | Emax (%) | EC50 (µM) | Emax (%) | EC50 (µM) | Emax (%) | EC50 (µM) | Emax (%) | EC50 (µM) | Emax (%) | |
| CB | 2.2 | 56 ± 11 | 2.4 | 112 ± 29 | 7.9 | 35 ± 8 | 2.7 | 58 ± 10 | >100 | 147 ± 23 | >100 | 219 ± 20 |
| SCG | 5.6 | 160 ± 14 | 4.6 | 82 ± 8 | 7.8 | 206 ± 19 | 1.9 | 89 ± 19 | 16.6 | 202 ± 37 | 70 | 96 ± 16 |
| CA | – | 18 ± 13 | 5.5 | 163 ± 31 | 1.9 | 31 ± 14 | 0.17 | 54 ± 20 | *54.5 | *180 ± 20 | *28.9 | *222 ± 38 |
EC50 and Emax calculated for IBMX 1–100 Mm.
CA, carotid artery; CB, carotid body; EC50, drug concentration that produces 50% of the maximal effect; Emax, mean maximal effect (%) observed in cAMP levels; IBMX, isobutylmethylxanthine; SCG, superior cervical ganglia.
Effects of PDE inhibitors on cAMP content in CAs
The effects of PDE inhibitors were also tested in rat CAs, both in normoxic and hypoxic conditions. As observed with the CBs, EHNA (0.1–100 µM) did not affect the cAMP levels in CAs either in normoxia or hypoxia. The effects of Ro 20-1724 (0.1–100 µM) and rolipram (0.1–100 µM) on cAMP in the CAs incubated with 20% O2/5% CO2 were similar and caused increases <40% (Figure 4A,B). Hypoxia clearly heightened the effects of both PDE4 selective inhibitors on cAMP content, specifically with higher concentrations of rolipram (>10 µM) and low concentrations of Ro 20-1724 (1 µM) (Figure 4A,B). The difference between cAMP accumulation in CAs during normoxia and hypoxia was particularly evident with rolipram (P < 0.0001, Figure 4A). Increases in cAMP accumulation caused by IBMX in CAs in both normoxic and hypoxic conditions were clearly more accentuated than those observed with Ro 20-1724 and rolipram (Figure 4C). These increases in cAMP in CAs induced by the non-specific PDE inhibitor, IBMX, apparently involve two mechanisms, one observed at low concentrations (<30 µM) which was heightened by hypoxia, and another observed with very high concentrations (0.1–1 mM) which was not modified by changes in oxygen (Figure 4C). The comparisons between the potency of the PDE4 inhibitors and the magnitude of their effects on cAMP are shown in Table 1 and expressed as EC50 and Emax. In this table, the effects on cAMP content in CAs induced by low (1–100 µM) concentrations of IBMX are shown separately in order to emphasize the effect of hypoxia observed in these experimental conditions.
Figure 4.
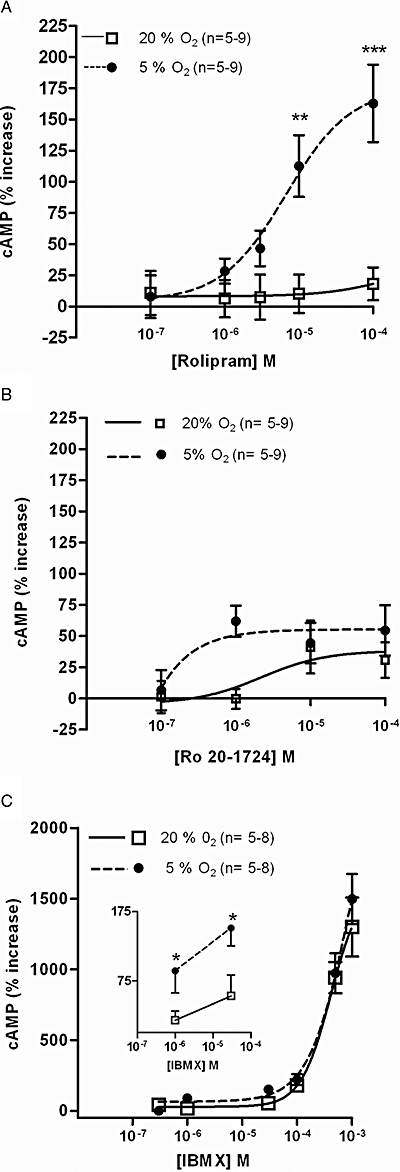
Effects of PDE4 inhibitors on cAMP levels in rat carotid arteries (CAs) incubated in normoxia [20%O2; 0% of effect corresponds to 0.19 ± 0.02 pmol·mg−1 (n= 12) ] and hypoxia [5%O2; 0% of effect corresponds to 0.11 ± 0.01 pmol·mg−1 (n= 11) ]. Concentration–response curve of (A) rolipram, (B) Ro 20-1724 and (C) isobutylmethylxanthine (IBMX) for effects on cAMP levels. **P < 0.01 and ***P < 0.001 two-way analysis of variance with Bonferroni's post hoc test. Values represent means ± SEM.
Effect of PDE inhibitors on cAMP content in SCG
In rat SCG, the effects of rolipram, Ro 20-1724, IBMX and EHNA on cAMP levels were also compared in normoxic and hypoxic conditions (Figure 5). Concentration-dependent increases in cAMP levels in normoxia were particularly evident for the PDE4 inhibitors: IBMX, Ro 20-1724 and rolipram (Figure 5A–C). In normoxia, the efficacy of the three inhibitors was similar (Figure 5A–C and Table 1). In contrast with the results obtained with CBs and CAs, in SCGs, hypoxia caused a statistically significant reduction in the effects of the PDE4 inhibitors on cAMP accumulation (Figure 5A–C).
Figure 5.
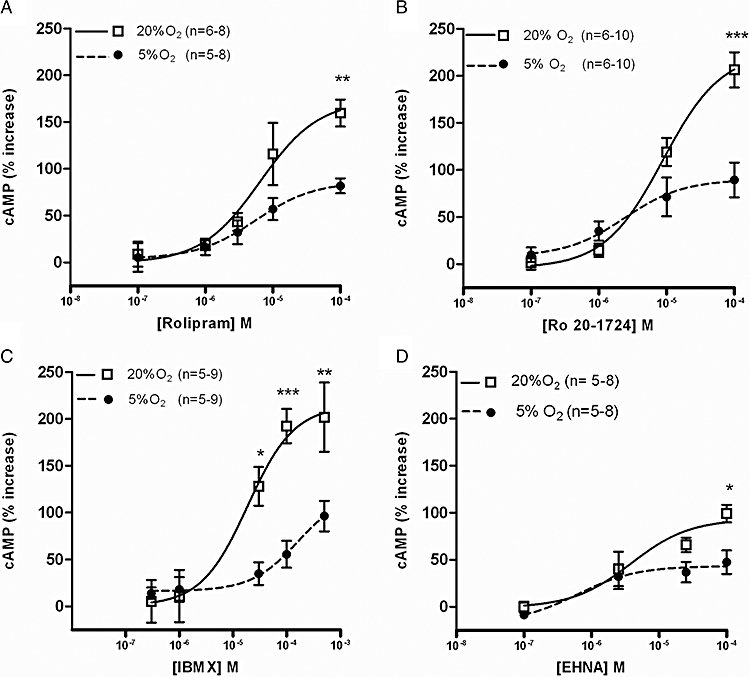
Concentration–response curves for the effects of (A) rolipram, (B) Ro 20-1724, (C) isobutylmethylxanthine (IBMX) and (D) erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) on cAMP levels in rat superior cervical ganglia in normoxia (20%O2) [0% effect − pmol 0.36 ± 0.03 pmol cAMP/mg tissue (n= 11) ] and in hypoxia (5%O2) [0% effect − 0.35 ± 0.04 pmol·cAMP·mg−1 tissue (n= 11) ]. *P < 0.05, **P < 0.01 and ***P < 0.001. Data values represent means ± SEM.
The effects of EHNA on cAMP accumulation in SCG were concentration-dependent (0.1–100 µM) and more pronounced (≈50%) than those obtained in CAs (<6%) and CBs (<10%). However, this effect was clearly less than those of the PDE4 inhibitors and apparently was not modified by hypoxia, except with concentrations higher than 10 µM (P < 0.05 comparing the effects of 100 µM in normoxia and hypoxia) (Figure 5D).
Discussion
A comparison between the effects of different PDE inhibitors in a functional study using tissues structurally related to peripheral oxygen chemosensors suggested that the in vivo effects of PDE inhibitors could be affected by acute hypoxia and demonstrated the presence of PDE4 isoforms in rat CBs, CAs and SCG. PDE2 appears to be absent in CBs and CAs and slightly active in SCG. The effects of PDE inhibitors on cAMP accumulation were increased by acute hypoxia in CBs and CAs but reduced in SCG. The differences in the sensitivity to hypoxia between cAMP accumulation induced by the two PDE4 selective inhibitors, rolipram and Ro 20-1724 indicate that they probably operate through different PDE4 isoforms.
This work was focused on PDE that use cAMP as a specific substract (PDE4, PDE7 and PDE8) and that are not modulated by cGMP, because cGMP levels are negligible and difficult to assess in a functional study of the CBs (Fidone et al., 1990). PDE4 isoenzymes are characterized by high affinity and specificity for cAMP, low KM (1.2–10 µM), insensitivity to Ca2+ and cGMP, selective inhibition by the archetypal rolipram and Ro 20-1724 (IC50 of 1 µM and 2 µM, respectively) (Bender and Beavo, 2006). No specific inhibitors are available for PDE8, but it is known that both PDE7 and PDE8 are insensitive to rolipram (Lugnier, 2006). IBMX is a commonly used tool to produce non-specific inhibition of PDEs1–5, but is devoid of effects on PDE7, PDE8 and PDE9 (Dousa, 1999; Lugnier, 2006). In addition to rolipram, Ro 20-1724 and IBMX, which allow the identification of PDE4, we have also investigated the effects of EHNA on cAMP accumulation in CBs, CAs and SCG. EHNA is a selective PDE2 (IC50= 2 µM, Podzuweit et al., 1995) inhibitor and PDE2 hydrolyses both cAMP and cGMP and is activated by cGMP (Lugnier, 2006). The rationale for including the effects of EHNA was that it also inhibits adenosine deaminase (Ki= 10−9 M) (Agarwal et al., 1977) and has been used as a tool to prevent adenosine degradation in experiments aimed to investigate the effects of adenosine receptors on cAMP in CBs (Monteiro et al., 1996).
Molecular characterizations of PDE have been extensively performed in systemic arteries and demonstrate that PDE3 and PDE4 families are the more active isoforms in aorta, coronary and renal arteries (Phillips et al., 2005). The characterization of PDE4 in CA was here performed for the first time because, so far, there was only one report, in bovine tissue, where three types of PDE were postulated: a PDE with high affinity for cAMP, two types of PDE with mixed affinity for cAMP and cGMP and a third type with high affinity for cGMP (Murtaugh and Bhalla, 1979). PDE2 is probably present in CAs, but, as found in pulmonary (Pauvert et al., 2002) and aortic arteries (Palmer et al., 1998) its activity is so much lower than, for instance, that of PDE4 (Phillips et al., 2005) that the consequences of its inhibition did not seem to be relevant in a pharmacological approach such as this. IBMX, apart from its ability to inhibit PDE non-selectively (Ki= 1–10 µM, Dousa, 1999) and IC50= 2–50 µM, (Bender and Beavo, 2006), is a xanthine able to antagonize adenosine receptors at the same order of concentration (Ki= 7–28 µM, Daly, 1991). In arteries, the adenosine receptors are mainly of the A2-receptor sub-type positively coupled to AC (http://www.iuphar-db.org/GPCR/index.jsp) and, as a consequence, the increase in cAMP accumulation after IBMX observed in CAs cannot be attributed to its antagonism of adenosine receptors.
An initial work regarding PDE isoforms in the CB appeared in 1977 (Hanbauer and Lovenberg, 1977), but no further studies are known and the characterization of PDE isoforms has never been performed in the CB. These authors provided evidence for a Ca++-dependent PDE in the CB and we have here characterized a PDE4, known as a Ca++-independent enzyme. In the CBs, the presence of active PDEs, other than PDE4 and sensitive to IBMX, was not conclusively shown because non-selective doses of the PDE4 inhibitors caused increases in cAMP of the same order of magnitude as those after IBMX and the contribution of the vascular component of the CB cannot be discarded. In addition, the selective PDE2 inhibitor EHNA (2.5–100 µM) did not modify cAMP concentrations in CBs. This is an interesting finding that validates the use of EHNA as a tool for manipulating endogenous concentrations of adenosine at the CB without apparent interference with PDE activities.
In the absence of PDE inhibitors, no changes in cAMP levels were observed in the CBs in response to hypoxia, although other studies have shown such changes previously (Pérez-Garcia et al., 1990). Species differences (rabbit, in the experiments by Pérez-Garcia et al., 1990) in the overall balance of AC/PDE could contribute to this difference in cAMP values obtained in response to changes in oxygen concentrations. In addition to species, other differences in the experimental procedures were found between the work of Pérez-Garcia et al. (1990) and the present one. In the earlier study, CBs were removed from CB bifurcations in vitro, allowed to recover in hyperoxic conditions and the effect of hypoxia was calculated from the comparison between cAMP concentrations obtained during incubations in 100% and 5% O2. We postulated that this latter issue could also contribute to the differences in cAMP accumulation because we have provided evidence that differences in the pre-incubation medium used to allow recovery of the preparations from surgical procedures did not cause significant changes in responses to further incubation.
The profile of responses to PDE inhibitors found in SCG, with the selective PDE inhibitors, rolipram and Ro 20-1724 more potent than IBMX, was compatible with a PDE 4 family predominantly active in cAMP hydrolysis. A similar potency for Ro 20-1724 was also found in a biochemical study that characterized a PDE4 in SCG in normoxic conditions (Giorgi et al., 1994). In this study, cGMP did not stimulate or inhibit PDE activity in SCG, suggesting that PDE 2 and PDE3 are absent or inactive (Giorgi et al., 1994). The results obtained here with the selective PDE2 inhibitor EHNA in SCG were not as clear as those obtained with CBs or CAs and did not allow the exclusion of PDE2 because a small concentration-dependent increase, attenuated by hypoxia, was observed with EHNA.
Hypoxia heightened the effects of PDE4 inhibitors on cAMP accumulation in CBs and CAs but, surprisingly, caused the opposite effect in SCG. In measuring cAMP, we cannot exclude the possibility that the effects of hypoxia include changes in AC. However, several data strongly support the hypothesis that oxygen concentrations selectively modify PDE activity. In the absence of PDE inhibitors, hypoxia did not increase basal cAMP values: they remained constant (SCG) or decreased (CAs and CBs). In addition, if the observed changes induced by hypoxia in cAMP concentrations were due to changes in AC activity, the differences found between the effects of hypoxia on different PDE inhibitors in the same preparation would be difficult to explain and changes in cAMP concentrations would be more pronounced with high concentrations of the PDE inhibitors when the catalytic pathway is completely blocked.
Increases in PDE activity induced by chronic hypoxia have been reported in a few studies (Maclean et al., 1997; Spoto et al., 1998; Hashimoto et al., 2004; Millen et al., 2006), but the effects of acute hypoxia are confined to the report of the increased activity of a Ca2+-dependent activator of cyclic nucleotide PDE in rat CB (Hanbauer and Lovenberg, 1977).
Currently, the PDE4 family represents the largest PDE family constituted by four genes A, B, C and D, but inhibitors targeting specific PDE splice variants are not available. Although rolipram and Ro 20-1724 do not apparently display isoform selectivity and present similar IC50 in purified enzyme preparations, some differences have been reported previously. Rolipram caused a much greater increase in isoprenaline-induced cAMP accumulation in rat pulmonary microvascular endothelial cells than Ro 20-1724 (Thompson et al., 2002). The rank order of potency for recombinant human PDE4C inhibition was rolipram > denbufyllin > Ro 20-1724 > IBMX (Engels et al., 1995). In general, rolipram is more potent than Ro 20-1724 in all (A, B, C and D) human PDE4 isoforms (Wang et al., 1997). In the experimental conditions of the present work, rolipram was devoid of effect in CAs in normoxia, and Ro 20-1724 was more potent than rolipram in the SCG and CA in hypoxia. The exact meaning of these findings is not clear but could suggest that specific PDE4 isoforms in the tissues are regulated in opposite directions by oxygen concentrations. In chronic experimental conditions, hypoxia increased the expression of PDE4 A, B and D in human pulmonary artery smooth muscle cells, but no overall increase in PDE4 activity was observed (Millen et al., 2006).
In conclusion, this pharmacological approach indicates that acute hypoxia could simultaneously potentiate cAMP production in systemic arteries induced by PDE4 inhibitors and reduce cAMP accumulation in sympathetic ganglia (SCG). The similarities between the characterization of PDE4 inhibitors at the CBs and CAs, under normoxia and hypoxia, did not support a specific role for cAMP in the oxygen-sensing machinery at the CB and suggests that probably no direct CB-mediated hyperventilatory adverse effects would be expected with administration of PDE4 inhibitors. Systemic vasodilator and, in general, smooth muscle-relaxing properties of specific PDE4 inhibitors, like rolipram, could be preferentially apparent in acute hypoxic conditions.
Acknowledgments
This work was supported by pluri-annual Centro de Estudos de Doenças Crónicas/Fundação para a Ciência e Tecnologia funding. The authors are grateful to the Department of Microbiology, Faculty of Medical Sciences for the valuable help in sample lyophilization.
Glossary
Abbreviations:
- AC
adenylate cyclase
- cAMP
3′-5′-cyclic adenosine monophosphate
- CA
carotid artery
- CB
carotid body
- EHNA
erythro-9-(2-hydroxy-3-nonyl)adenine
- IBMX
isobutylmethylxanthine
- PDE
phosphodiesterase
- SCG
superior cervical ganglia
References
- Agarwal RP, Spector T, Parks RE. Tight-binding inhibitors-IV. Inhibition of adenosine deaminases by various inhibitors. Biochem Pharmacol. 1977;26:359–367. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaraz L, Perez-Garcia MT, Gonzalez C. Presence of D1 receptors in the rabbit carotid body. Neurosci Lett. 1991;132(2):259–262. doi: 10.1016/0304-3940(91)90315-k. [DOI] [PubMed] [Google Scholar]
- Batuca JR, Monteiro TC, Monteiro EC. Contribution of dopamine D2 receptors for the cAMP levels at the carotid body. Adv Exp Med Biol. 2003;536:367–373. doi: 10.1007/978-1-4419-9280-2_48. [DOI] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Chen J, Dinger B, Fidone SJ. cAMP production in rabbit carotid body: role of adenosine. J Appl Physiol. 1997;82(6):1771–1775. doi: 10.1152/jappl.1997.82.6.1771. [DOI] [PubMed] [Google Scholar]
- Conde SV, Monteiro EC. Hypoxia induces adenosine release from rat carotid body. J Neurochem. 2004;89:1148–1156. doi: 10.1111/j.1471-4159.2004.02380.x. [DOI] [PubMed] [Google Scholar]
- Conde SV, Monteiro EC. Activation of nicotinic Ach receptors with α4 subunits induces adenosine release at the rat carotid body. Br J Pharmacol. 2006;147:783–789. doi: 10.1038/sj.bjp.0706676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde SV, Obeso A, Vicario I, Rigual R, Rocher A, Gonzalez C. Caffeine inhibition of rat carotid body chemoreceptors is mediated by A2A and A2B adenosine receptors. J Neurochem. 2006;98(2):616–628. doi: 10.1111/j.1471-4159.2006.03912.x. [DOI] [PubMed] [Google Scholar]
- Daly JW. Caffeine analogs: structure–activity relationships at adenosine receptors. Pharmacology. 1991;42(6):309–321. doi: 10.1159/000138813. [DOI] [PubMed] [Google Scholar]
- Delpiano MA, Acker H. Hypoxia increases the cyclic AMP content of the cat carotid body in vitro. J Neurochem. 1991;57(1):291–297. doi: 10.1111/j.1471-4159.1991.tb02127.x. [DOI] [PubMed] [Google Scholar]
- Dousa TP. Cyclic-3',5'- nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int. 1999;55:29–62. doi: 10.1046/j.1523-1755.1999.00233.x. [DOI] [PubMed] [Google Scholar]
- Engels P, Sullivan M, Muller T, Lubbert H. Molecular cloning and functional expression in yeast of a human cAMP-specific phosphodiesterase subtype (PDE IV-C) FEBS Lett. 1995;358:305–310. doi: 10.1016/0014-5793(94)01460-i. [DOI] [PubMed] [Google Scholar]
- Fidone S, Gonzalez C, Dinger B, Stensaas L. Transmitter dynamics in the carotid body. In: Acker H, Trzebski A, O'Reagan RG, editors. Chemoreceptors and Chemoreceptor Reflex. New York: Plenum Press; 1990. pp. 3–13. [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Giorgi M, Squitti R, Bonse P, Paggi P, Toschi G. Activities of 3'5'cyclic nucleotide phosphodiesterases in the superior cervical ganglion of rat: characterization, compartmentalization and observations in young and old animals. Neurochem Int. 1994;25:493–500. doi: 10.1016/0197-0186(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Hanbauer I, Lovenberg W. Presence of a calcium 2+-dependent activator of cyclic nucleotide phosphodiesterase in rat carotid body: effects of hypoxia. Neuroscience. 1977;2:603–607. doi: 10.1016/0306-4522(77)90057-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Sugiyama A, Taguchi S. Myosin heavy chain isoforms expression and cyclic AMP concentrations in hypoxia-induced hypertrophied right ventricle in rats. Comp Biochem Physiol B Biochem Mol Biol. 2004;138(4):365–370. doi: 10.1016/j.cbpc.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Jeon YH, Heo YS, Kim YL, Lee TG, Cho JM. Phosphodiesterases: overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell Mol Life Sci. 2005;62:1198–1220. doi: 10.1007/s00018-005-4533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutics agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- McDonald DM, Mitchell RA. The innervation of glomus cells, ganglion cells and blood vessels in the rat carotid body: a quantitative ultrastructural analysis. J Neurocytol. 1975;4:177–230. [Google Scholar]
- Maclean MR, Johnston ED, Mcculloch KM, Pooley L, Houslay MD, Sweeney G. Phosphodiesterases isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J Pharmacol Exp Ther. 1997;283(2):619–624. [PubMed] [Google Scholar]
- Millen J, Maclean MR, Houslay MD. Hypoxia-induced remodelling of PDE4 isoform expression and cAMP handling in human pulmonary artery smooth muscle cells. Eur J Cell Biol. 2006;85(7):679–691. doi: 10.1016/j.ejcb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Mir AK, McQueen DS, Pallot DJ, Nahorski SR. Direct biochemical and neuropharmacological identification of dopamine D2-receptors in the rabbit carotid body. Brain Res. 1984;291(2):273–283. doi: 10.1016/0006-8993(84)91259-9. [DOI] [PubMed] [Google Scholar]
- Mokashi A, Lahiri S. Aortic and carotid body chemoreception in prolonged hyperoxia in the cat. Respir Physiol. 1991;86:233–243. doi: 10.1016/0034-5687(91)90083-u. [DOI] [PubMed] [Google Scholar]
- Mokashi A, Di Giulio C, Morelli L, Lahiri S. Chronic hyperoxic effects on cat carotid body catecholamines and structure. Respir Physiol. 1994;97:25–32. doi: 10.1016/0034-5687(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Monteiro EC, Vera-Cruz P, Monteiro TC, Silva e Sousa MA. Adenosine increases the cAMP content of the rat carotid body in vitro. Adv Exp Med Biol. 1996;410:299–303. doi: 10.1007/978-1-4615-5891-0_45. [DOI] [PubMed] [Google Scholar]
- Murtaugh TJ, Bhalla RC. Multiple forms of cyclic nucleotide phosphodiesterase from bovine carotid artery smooth muscle. Arch Biochem Biophys. 1979;2:465–474. doi: 10.1016/0003-9861(79)90298-4. [DOI] [PubMed] [Google Scholar]
- Nunes AR, Batuca JR, Monteiro EC. Functional characterization of phosphodiesterases 4 in the rat carotid body: effect of oxygen concentrations. In: González C, Nurse C, Peers C, editors. The Arterial Chemoreceptors. New York: Springer; 2009. pp. 129–136. [DOI] [PubMed] [Google Scholar]
- Palmer D, Tsoi K, Maurice DH. Synergetic inhibition of vascular muscle smooth cell migration by phosphodiesterase 3 and phosphodiesterase 4 inhibitors. Circ Res. 1998;82:852–861. doi: 10.1161/01.res.82.8.852. [DOI] [PubMed] [Google Scholar]
- Pauvert O, Salvail D, Rousseau E, Lugnier C, Marthan R, Savineau JP. Characterization of cyclic nucleotide phosphodiesterase isoforms in the media layer of the main pulmonary artery. Biochem Pharmacol. 2002;63:1763–1772. doi: 10.1016/s0006-2952(02)00919-x. [DOI] [PubMed] [Google Scholar]
- Pérez-Garcia MT, Almaraz L, Gonzalez C. Effects of different types of stimulation on cyclic AMP content of the rabbit carotid body: functional significance. J Neurochem. 1990;57:1287–1293. doi: 10.1111/j.1471-4159.1990.tb03137.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Garcia MT, Almaraz L, Gonzaléz C. Cyclic AMP modulates differentially the release of dopamine induced by hypoxia and other stimuli and increased dopamine synthesis in the rabbit carotid body. J Neurochem. 1991;57:1992–2000. doi: 10.1111/j.1471-4159.1991.tb06414.x. [DOI] [PubMed] [Google Scholar]
- Phillips PG, Long L, Wilkins MR, Morrell NW. cAMP phosphodiesterase inhibitors potentiate effects of prostacyclin analogs in hypoxic pulmonary vascular remodeling. Am J Physiol. 2005;288:L103–L115. doi: 10.1152/ajplung.00095.2004. [DOI] [PubMed] [Google Scholar]
- Podzuweit T, Nennstiel P, Muller A. Isozyme selective inhibition of cGMP-stimulated cyclic nucleotide phosphodiesterases by erythro-9-(2-hydroxy-3-nonyl) adenine. Cell Signal. 1995;7:733–738. doi: 10.1016/0898-6568(95)00042-n. [DOI] [PubMed] [Google Scholar]
- Spoto G, Di Giulio C, Contento A, Di Stilio M. Hypoxia effect on blood phosphodiesterase activity in young and old rats. Pharm Lett Life Sci. 1998;63:349–353. doi: 10.1016/s0024-3205(98)00513-x. [DOI] [PubMed] [Google Scholar]
- Thompson WJ, Ashikaga T, Kelly JJ, Liu L, Zhu B, Vemavarapau L, et al. Regulation of cyclic AMP in rat pulmonary microvascular endothelial cells by rolipram-sensitive cyclic AMP phosphodiesterase (PDE4) Biochem Pharmacol. 2002;63:797–807. doi: 10.1016/s0006-2952(01)00914-5. [DOI] [PubMed] [Google Scholar]
- Torbati D, Sherpa AK, Lahiri S, Mokashi A, Albertine KH, Di Giulio C. Hyperbaric oxygenation alters carotid body ultrastructure and function. Respir Physiol. 1993;92:183–196. doi: 10.1016/0034-5687(93)90037-b. [DOI] [PubMed] [Google Scholar]
- Wang P, Myers JG, Wu P, Cheewatrakoolpong B, Egan RW, Billah M. Expression, purification and characterization of human cAMP-specific phosphodiesterase PDE4 Subtypes A,B,C and D. Biochem Biophys Res Commun. 1997;234:320–324. doi: 10.1006/bbrc.1997.6636. [DOI] [PubMed] [Google Scholar]


