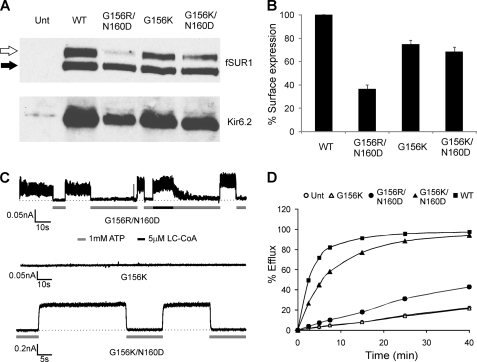
FIGURE 4.
Restoration of ion conduction in G156R or G156K mutant channels by a second site mutation N160D. A, shown are Western blots of SUR1 and Kir6.2 in COSm6 cells co-transfected with cDNAs for fSUR1 and one of the following Kir6.2s: WT, G156R/N160D, G156K, or G156K/N160D. B, surface expression of various channels was quantified using the chemiluminescence assay described under “Materials and Methods.” C, representative traces are shown of inside-out patch clamp recordings from cells expressing different mutant channels. In contrast to the G156R mutant shown in Fig. 1C, ATP-sensitive currents were readily detected from cells expressing the G156R/N160D mutant. Some activation by LC-CoA was also apparent based on the slow inhibition by ATP after LC-CoA exposure. The G156K mutation also abolished channel activity, and this defect was similarly overcome by introducing the second mutation N160D. D, 86Rb+ efflux assays show activation of the G156R/N160D and G156K/N160D mutant channels by metabolic inhibition as seen for WT channels, albeit to a lesser extent likely due to differences in expression levels and gating properties (see also Fig. 8 for ATP and MgADP responses). In contrast, no channel activity was detected for the G156K mutant, as with the G156R mutant shown in Fig. 1.