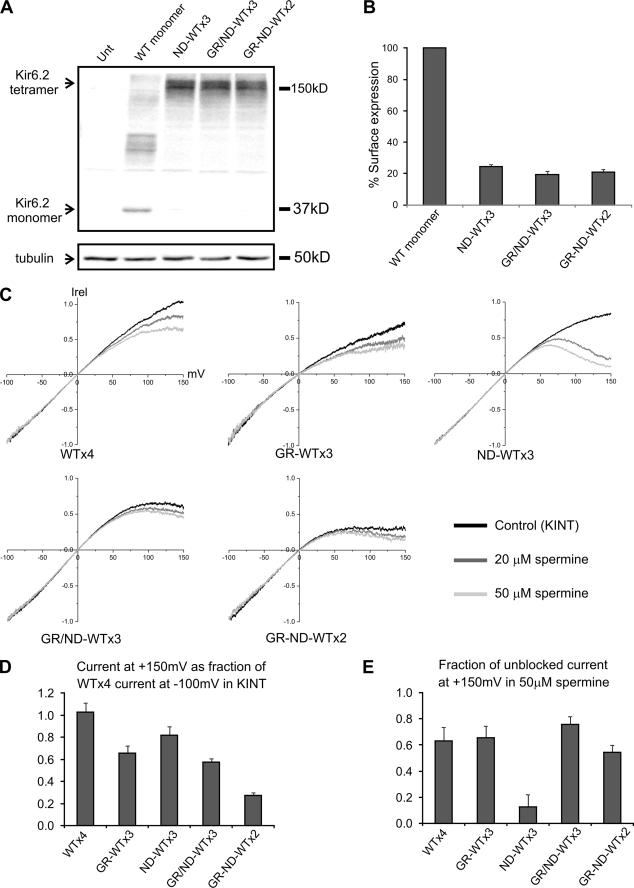
FIGURE 7.
Intra- and intersubunit interactions between G156R and N160D in the Kir6.2 tetramer. A, shown is a Western blot of Kir6.2 monomer and various tandem tetramers expressed in COS cells. All cells were co-transfected with SUR1, and the tubulin blot was shown as the loading controls. B, chemiluminescence assay results show surface expression of the various mutant tandem tetramer channels, although at a much reduced level compared with channels formed by co-expressing SUR1 and Kir6.2 monomer. Note in this figure that GR denotes G156R, and ND denotes N160D. GR/ND refers to G156R and N160D in the same subunit, and GR-ND refers to G156R and N160D in adjacent subunits in the Kir6.2 tandem tetramer. C, inside-out patches containing various tandem tetramer channels were subjected to a voltage-ramp protocol (+150 to −100 mV) in control K-INT solution or K-INT solution containing 20 or 50 μm spermine. Averaged current-voltage profiles of the channels (from four-six patches) are shown. Currents were normalized (Irel) to that seen at −100 mV. D, current of the various mutants at +150 mV in K-INT control solution was normalized to that of WT tandem tetramer (denoted as WT×4) at −100 mV in K-INT to show the spermine-independent difference caused by the mutations. E, Current observed at +150 mV in the presence of 50 μm spermine for each channel was shown as the fraction of current observed at the same membrane potential in K-INT solution for the respective channel.