FIGURE 8.
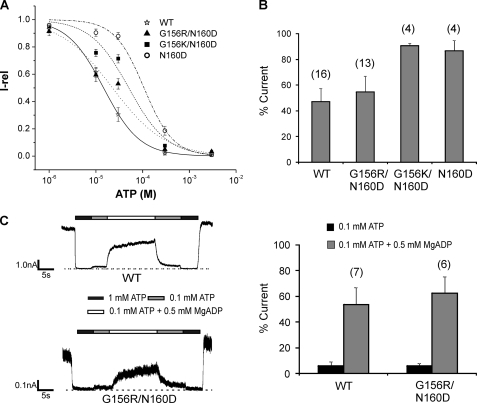
Nucleotide sensitivities of G156R/N160D charge-pair mutant channels. A, ATP dose-response curves obtained by fitting the data points to the modified Hill equation (Irel = 1/(1+ ([ATP]/IC50)H)), where Irel is the current relative to that seen in the absence of ATP, IC50 is [ATP] necessary for half-maximal inhibition of channel activity, and H the Hill coefficient. Recordings were made in K-INT/1 mm EDTA solution to minimize rundown (38). Error bars represent S.E. of 5–6 patches. The IC50 values are as shown in Table 1. The H values are WT = 1.2 ± 0.2, G156R/N160D = 0.8 ± 0.2, G156K/N160D = 1.2 ± 0.5, and N160D = 1.4 ± 0.2. B, ATP sensitivity was also assessed by comparing channel activity at 20 μm ATP. The number of patches for each channel type is shown above the bars. There was no significant difference between WT and G156R/N160D (p > 0.05), but a significant difference was observed between WT and G156K/N160D or N160D (p < 0.01). C, left, representative traces of WT and G156R/N160D channels show channel response to MgADP. Patches were exposed to various concentrations of ATP and ADP as indicated by the bars above the recordings. Free [Mg2+] was 1 mm in all solutions. Right, quantification is shown of channel response to ATP and MgADP. Currents in 0.1 mm ATP or 0.1 mm ATP plus 0.5 mm ADP were normalized to currents in nucleotide-free solution. Again, there is no significant difference (6–7 patches) in MgADP response between WT and G156R/N160D (p > 0.05).