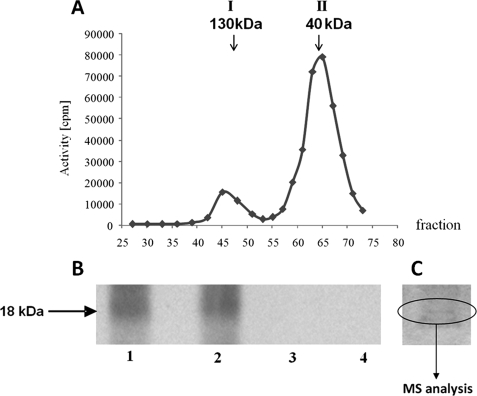
FIGURE 1.
Analysis of CK2 activity in maize seedlings. A, separation on Sephadex G-100 of two peaks (I130 and II40) of the kinase activity. CK2 activity was determined as described under “Experimental Procedures” using casein as a substrate (see “In Vitro Phosphorylation Assay”). B, phosphorylation of proteins in peak II40 was performed in 50 μl of reaction mixture containing 50 μm ATP, 20 mm Tris-HCl (pH 7.5), 20 mm MgCl2; alone (lane 1), in the presence of 10 μm staurosporine (lane 2), 10 μm heparin (lane 3), or 50 μm 4,5,6,7-tetrabromobenzotriazole (lane 4). [γ-32P]ATP was used for detection of phosphorylation, whereas nonradioactive ATP was used for MS analysis. After 20 min of incubation at 30 °C the reaction was stopped by addition of Laemmli buffer, heated at 95 °C for 5 min, and subjected to SDS-PAGE. In the case of proteins phosphorylated with [γ-32P]ATP, the gel was dried and exposed to autoradiography. C, SDS-PAGE of proteins from peak II40. For MS analysis, the appropriate protein band (18 kDa) stained with Coomassie Brilliant Blue was cut from the wet gel.