FIGURE 3.
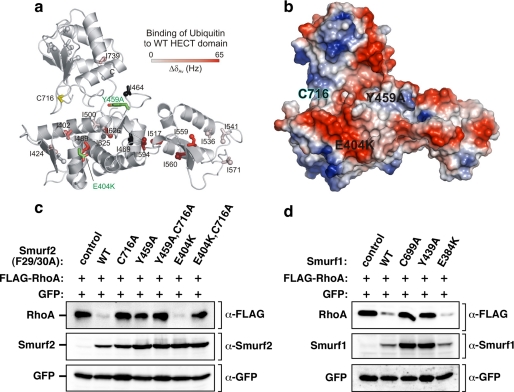
The ubiquitin binding surface of Smurfs is required for targeting RhoA in cells. a, residues experiencing significant chemical shift changes were mapped onto the structure of the Smurf2 HECT domain (PDB ID 1ZVD) and colored with a linear gradient from white (ΔδAv ≤ 0.0 Hz) to red (ΔδAv = 65 Hz). Spheres represent the δ1 carbon atoms of affected Ile residues. Ile residues with missing resonance assignments (I464, I469, and I625) are shown in black. The active site cysteine (Cys-716) and the mutations interfering with ubiquitin binding (E404K and Y459A) are highlighted in yellow and green. b, shown is an electrostatic surface potential representation of the Smurf2 HECT domain in the same orientation as in Fig. 4a, with the positions of the E404K and Y459A mutations labeled on the HECT domain surface. c, mutation in the Smurf UBS interferes with RhoA targeting in cells. HEK293T cells were transiently transfected with FLAG-tagged RhoA in combination with empty vector, F29A/F30A, or F29A/F30A catalytically inactive Myc-tagged Smurf2 with an otherwise WT HECT domain or a HECT domain harboring the indicated UBS mutants. RhoA and Smurf2 steady-state levels in total cell lysates were determined by immunoblotting with α-FLAG and α-Smurf2 antibody (top panel and bottom panel), respectively. d, mutation in the Smurf1 UBS interferes with RhoA targeting in cells. HEK293T cells were transiently transfected with FLAG-tagged RhoA in combination with empty vector or Myc-tagged Smurf1 that was WT, C699A, and Y439A, or E384K (which correspond, respectively, to Tyr-459 and Glu-404 in Smurf2). RhoA and Smurf1 steady-state levels in total cell lysates were determined by immunoblotting with α-FLAG and α-Smurf1 antibody (top panel and bottom panel), respectively.