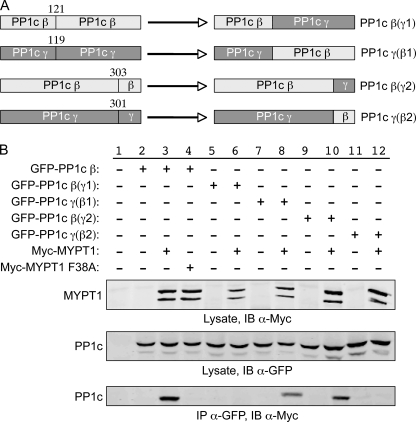
FIGURE 4.
The C terminus of PP1c β does not mediate the isoform specificity of interaction with MYPT1. A, structure of PP1 chimera constructs. Based on the alignment of the three human PP1c isoforms, two sets of chimera constructs were generated as shown for PP1c β and PP1c γ. In the first set, PP1c was divided in half, and the C-terminal halves of PP1c β and PP1c γ were exchanged. These constructs were denoted PP1c β(γ1) and PP1c γ(β1). For the second set of constructs, the extreme C termini (last 30 amino acids) of PP1c β and PP1c γ were exchanged. These constructs were denoted PP1c β(γ2) and PP1c γ(β2). B, HeLa cells were transiently co-transfected with the GFP-tagged PP1c wild-type or chimeric plasmids and Myc-tagged wild-type or mutant MYPT1 as indicated. 48 h later, the cells were lysed, and Western blotting was performed against the corresponding proteins using the appropriate antibodies. Immunoprecipitation assays were performed as described in Fig. 1.