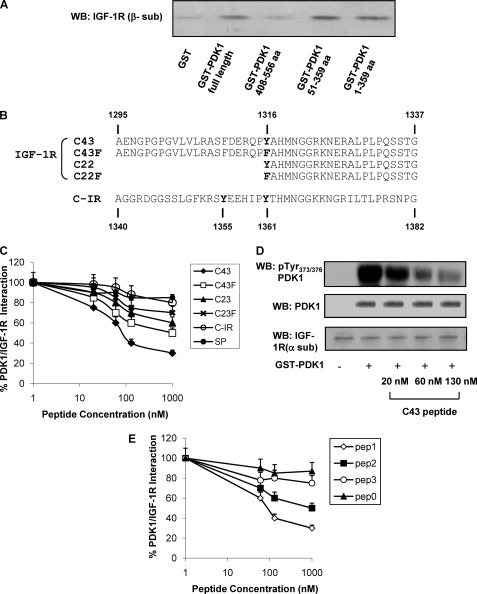
FIGURE 2.
In vitro interaction of IGF-1R with PDK1. A, IGF-1R was partially purified from NIH3T3IGF-1R by wheat germ agglutinin affinity chromatography and subjected to pulldown using full-length GST-PDK1 and deletion mutants (amino acids (aa) 1–359, 51–359, and 408–556). Equal amounts of IGF-1R were incubated with each fusion protein and then eluted and analyzed by 7.5% SDS-PAGE; IGF-1R bound to fusion proteins was detected by Western blotting (WB) using anti-β-subunit IGF-1R antibodies. B, amino acid sequence alignment of synthetic peptides corresponding to IGF-1R C terminus fragment (C43; C22), to a mutated version, in which Tyr1316 has been substituted with Phe (C43F; C22F); and to C-IR. C, partially purified IGF-1R was incubated for 2 h at 4 °C with Sepharose-GST-PDK1 in the presence or in the absence of synthetic peptides (C43, C43F, C22, C22F, C-IR, and SP) at increasing concentrations as indicated. Pulled-down proteins were blotted with anti-IGF-1R (β-subunit) antibody, and the results were quantitated by laser densitometry. Error bars indicate mean ± S.D. D, equal amounts of purified IGF-1R were stimulated with 100 ng/ml IGF-1, as described under “Experimental Procedures,” and incubated or not with GST-PDK1 in the presence of increasing concentrations of C43, as indicated. Proteins were blotted with pTyr373/376-PDK1 antibodies. Membranes were reblotted with PDK1 and IGF-1R (α-subunit) antibodies, as shown on the bottom. Blots were revealed by ECL and autoradiography. E, partially purified IGF-1R was incubated for 2 h at 4 °C with Sepharose-GST-PDK1 in the presence or in the absence of synthetic peptides (pep1, pep2, pep3, and pep0) at increasing concentrations as indicated. Pulled-down proteins were blotted with anti-IGF-1R (β-subunit) antibody, and the results were quantitated by laser densitometry. All the autoradiographs shown are representative of at least four independent experiments.